Multiple myeloma: patient outcomes in real-world practice
- PMID: 27411022
- PMCID: PMC5096152
- DOI: 10.1111/bjh.14213
Multiple myeloma: patient outcomes in real-world practice
Abstract
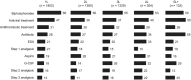
With increasing number of therapies available for the treatment of multiple myeloma, it is timely to examine the course of patients' journeys. We investigated patient characteristics, treatment durations and outcomes, and symptom burden across the treatment pathway in Belgium, France, Germany, Italy, Spain, Switzerland and the UK. In total, 435 physicians retrospectively reviewed 4997 patient charts. Profiles of patients diagnosed with multiple myeloma during the last 12 months were similar across countries; bone pain was the most common presentation. Median duration of first-line therapy was 6 months, followed by a median treatment-free interval of 10 months; both these decreased with increasing lines of therapy, as did time to progression. Depth of response, as assessed by the treating physician, also decreased with each additional line of therapy: 74% of patients achieved at least a very good partial response at first line, compared with only 11% at fifth line. Deeper responses were associated with longer time to progression, although these were physician-judged. Toxicities and co-morbidities increased with later treatment lines, and were more likely to have led to discontinuation of treatment. These real-world data provide an insight into patient outcomes and treatment decisions being made in clinical practice.
Keywords: depth of response; duration of therapy; multiple myeloma; patient chart review; real-world practice.
© 2016 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd.
Figures







References
-
- Acaster, S. , Gaugris, S. , Velikova, G. , Yong, K. & Lloyd, A.J. (2013) Impact of the treatment‐free interval on health‐related quality of life in patients with multiple myeloma: a UK cross‐sectional survey. Supportive Care in Cancer, 21, 599–607. - PubMed
-
- Benboubker, L. , Dimopoulos, M.A. , Dispenzieri, A. , Catalano, J. , Belch, A.R. , Cavo, M. , Pinto, A. , Weisel, K. , Ludwig, H. , Bahlis, N. , Banos, A. , Tiab, M. , Delforge, M. , Cavenagh, J. , Geraldes, C. , Lee, J.J. , Chen, C. , Oriol, A. , de la Rubia, J. , Qiu, L. , White, D.J. , Binder, D. , Anderson, K. , Fermand, J.P. , Moreau, P. , Attal, M. , Knight, R. , Chen, G. , Van Oostendorp, J. , Jacques, C. , Ervin‐Haynes, A. , Avet‐Loiseau, H. , Hulin, C. & Facon, T. (2014) Lenalidomide and dexamethasone in transplant‐ineligible patients with myeloma. New England Journal of Medicine, 371, 906–917. - PubMed
-
- Carpenter, J. & Bithell, J. (2000) Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Statistics in Medicine, 19, 1141–1164. - PubMed
-
- Dimopoulos, M. , Spencer, A. , Attal, M. , Prince, H.M. , Harousseau, J.L. , Dmoszynska, A. , San Miguel, J. , Hellmann, A. , Facon, T. , Foa, R. , Corso, A. , Masliak, Z. , Olesnyckyj, M. , Yu, Z. , Patin, J. , Zeldis, J.B. & Knight, R.D. (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. New England Journal of Medicine, 357, 2123–2132. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

