BK virus encephalopathy and sclerosing vasculopathy in a patient with hypohidrotic ectodermal dysplasia and immunodeficiency
- PMID: 27411570
- PMCID: PMC4944483
- DOI: 10.1186/s40478-016-0342-3
BK virus encephalopathy and sclerosing vasculopathy in a patient with hypohidrotic ectodermal dysplasia and immunodeficiency
Abstract
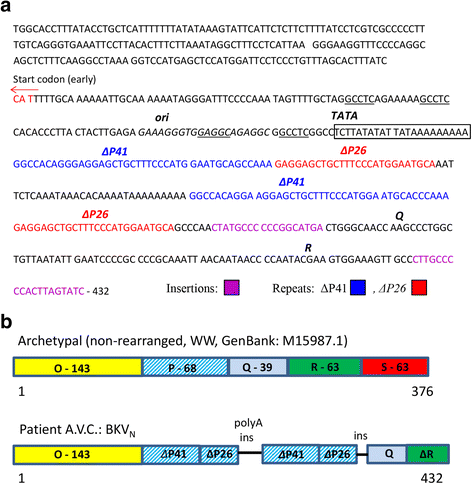
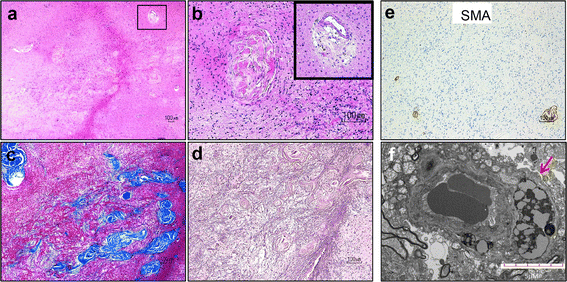
Human BK polyomavirus (BKV) is reactivated under conditions of immunosuppression leading most commonly to nephropathy or cystitis; its tropism for the brain is rare and poorly understood. We present a unique case of BKV-associated encephalopathy in a man with hypohidrotic ectodermal dysplasia and immunodeficiency (HED-ID) due to IKK-gamma (NEMO) mutation, who developed progressive neurological symptoms. Brain biopsy demonstrated polyomavirus infection of gray and white matter, with predominant involvement of cortex and distinct neuronal tropism, in addition to limited demyelination and oligodendroglial inclusions. Immunohistochemistry demonstrated polyoma T-antigen in neurons and glia, but expression of VP1 capsid protein only in glia. PCR analysis on both brain biopsy tissue and cerebrospinal fluid detected high levels of BKV DNA. Sequencing studies further identified novel BKV variant and disclosed unique rearrangements in the noncoding control region of the viral DNA (BKVN NCCR). Neuropathological analysis also demonstrated an unusual form of obliterative fibrosing vasculopathy in the subcortical white matter with abnormal lysosomal accumulations, possibly related to the patient's underlying ectodermal dysplasia. Our report provides the first neuropathological description of HED-ID due to NEMO mutation, and expands the diversity of neurological presentations of BKV infection in brain, underscoring the importance of its consideration in immunodeficient patients with unexplained encephalopathy. We also document novel BKVN NCCR rearrangements that may be associated with the unique neuronal tropism in this patient.
Keywords: BK virus; Ectodermal dysplasia; Encephalopathy; Fibrosing vasculopathy; HED-ID; IKK-gamma; NF-kappa-B essential modulator (NEMO); Polyomavirus.
Figures



Similar articles
-
Hypohidrotic ectodermal dysplasia, osteopetrosis, lymphedema, and immunodeficiency in an infant with multiple opportunistic infections.Pediatr Dermatol. 2014 Nov-Dec;31(6):716-21. doi: 10.1111/pde.12103. Epub 2013 Feb 14. Pediatr Dermatol. 2014. PMID: 23405946
-
Frequent somatic mosaicism of NEMO in T cells of patients with X-linked anhidrotic ectodermal dysplasia with immunodeficiency.Blood. 2012 Jun 7;119(23):5458-66. doi: 10.1182/blood-2011-05-354167. Epub 2012 Apr 19. Blood. 2012. PMID: 22517901
-
A novel NEMO gene mutation causing osteopetrosis, lymphoedema, hypohidrotic ectodermal dysplasia and immunodeficiency (OL-HED-ID).Eur J Pediatr. 2010 Nov;169(11):1403-7. doi: 10.1007/s00431-010-1206-7. Epub 2010 May 21. Eur J Pediatr. 2010. PMID: 20499091
-
Noncoding control region of naturally occurring BK virus variants: sequence comparison and functional analysis.Virus Genes. 1995;10(3):261-75. doi: 10.1007/BF01701816. Virus Genes. 1995. PMID: 8560788 Review.
-
BK virus-associated renal problems--clinical implications.Pediatr Nephrol. 2003 Aug;18(8):743-8. doi: 10.1007/s00467-003-1184-3. Epub 2003 Jun 12. Pediatr Nephrol. 2003. PMID: 12802640 Review.
Cited by
-
Comparison of qPCR with ddPCR for the Quantification of JC Polyomavirus in CSF from Patients with Progressive Multifocal Leukoencephalopathy.Viruses. 2022 Jun 8;14(6):1246. doi: 10.3390/v14061246. Viruses. 2022. PMID: 35746716 Free PMC article.
-
Prevalence of BK and JC Polyomaviruses among Patients with Breast Cancer.Asian Pac J Cancer Prev. 2024 Mar 1;25(3):821-827. doi: 10.31557/APJCP.2024.25.3.821. Asian Pac J Cancer Prev. 2024. PMID: 38546065 Free PMC article.
-
The case for BK polyomavirus as a cause of bladder cancer.Curr Opin Virol. 2019 Dec;39:8-15. doi: 10.1016/j.coviro.2019.06.009. Epub 2019 Jul 20. Curr Opin Virol. 2019. PMID: 31336246 Free PMC article. Review.
-
Ectodysplasin A (EDA) Signaling: From Skin Appendage to Multiple Diseases.Int J Mol Sci. 2022 Aug 10;23(16):8911. doi: 10.3390/ijms23168911. Int J Mol Sci. 2022. PMID: 36012178 Free PMC article. Review.
-
JC polyoma viruria associates with protection from chronic kidney disease independently from apolipoprotein L1 genotype in African Americans.Nephrol Dial Transplant. 2018 Nov 1;33(11):1960-1967. doi: 10.1093/ndt/gfx368. Nephrol Dial Transplant. 2018. PMID: 29420808 Free PMC article.
References
-
- Barcena-Panero A, Echevarria JE, Van Ghelue M, Fedele G, Royuela E, Gerits N, Moens U. BK polyomavirus with archetypal and rearranged non-coding control regions is present in cerebrospinal fluids from patients with neurological complications. J Gen Virol. 2012;93:1780–1794. doi: 10.1099/vir.0.042143-0. - DOI - PubMed
-
- Barcena-Panero A, Van Ghelue M, Khan MT, Echevarria JE, Fedele G, Moens U. BK virus-associated infection in cerebrospinal fluid of neurological patients and mutation analysis of the complete VP1 gene in different patient groups. J Cell Physiol. 2012;227:136–145. doi: 10.1002/jcp.22711. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

