Glucose modulates food-related salience coding of midbrain neurons in humans
- PMID: 27411574
- PMCID: PMC6867299
- DOI: 10.1002/hbm.23316
Glucose modulates food-related salience coding of midbrain neurons in humans
Abstract
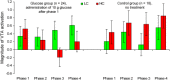
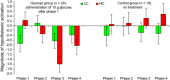
Although early rat studies demonstrated that administration of glucose diminishes dopaminergic midbrain activity, evidence in humans has been lacking so far. In the present functional magnetic resonance imaging study, glucose was intravenously infused in healthy human male participants while seeing images depicting low-caloric food (LC), high-caloric food (HC), and non-food (NF) during a food/NF discrimination task. Analysis of brain activation focused on the ventral tegmental area (VTA) as the origin of the mesolimbic system involved in salience coding. Under unmodulated fasting baseline conditions, VTA activation was greater during HC compared with LC food cues. Subsequent to infusion of glucose, this difference in VTA activation as a function of caloric load leveled off and even reversed. In a control group not receiving glucose, VTA activation during HC relative to LC cues remained stable throughout the course of the experiment. Similar treatment-specific patterns of brain activation were observed for the hypothalamus. The present findings show for the first time in humans that glucose infusion modulates salience coding mediated by the VTA. Hum Brain Mapp 37:4376-4384, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging; glucose modulation; mesolimbic system; obesity; reward; salience coding; ventral tegmental area.
© 2016 Wiley Periodicals, Inc.
Figures


Similar articles
-
Glucose Modulates Human Ventral Tegmental Activity in Response to Sexual Stimuli.J Sex Med. 2018 Jan;15(1):20-28. doi: 10.1016/j.jsxm.2017.11.014. J Sex Med. 2018. PMID: 29289371
-
Theta-burst modulation of mid-ventrolateral prefrontal cortex affects salience coding in the human ventral tegmental area.Appetite. 2018 Apr 1;123:91-100. doi: 10.1016/j.appet.2017.12.015. Epub 2017 Dec 13. Appetite. 2018. PMID: 29247796
-
Differential involvement of brainstem noradrenergic and midbrain dopaminergic nuclei in cognitive control.Hum Brain Mapp. 2016 Jun;37(6):2305-18. doi: 10.1002/hbm.23173. Epub 2016 Mar 11. Hum Brain Mapp. 2016. PMID: 26970351 Free PMC article.
-
Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain.Biol Psychiatry. 2013 Aug 1;74(3):172-9. doi: 10.1016/j.biopsych.2012.12.023. Epub 2013 Feb 16. Biol Psychiatry. 2013. PMID: 23419544 Clinical Trial.
-
Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: Historical perspective.Brain Res. 2016 Aug 15;1645:68-70. doi: 10.1016/j.brainres.2015.12.041. Epub 2015 Dec 28. Brain Res. 2016. PMID: 26731335 Review.
Cited by
-
The Neurobiology of Eating Behavior in Obesity: Mechanisms and Therapeutic Targets: A Report from the 23rd Annual Harvard Nutrition Obesity Symposium.Am J Clin Nutr. 2023 Jul;118(1):314-328. doi: 10.1016/j.ajcnut.2023.05.003. Epub 2023 May 4. Am J Clin Nutr. 2023. PMID: 37149092 Free PMC article.
-
The influence of the subcortex and brain stem on overeating: How advances in functional neuroimaging can be applied to expand neurobiological models to beyond the cortex.Rev Endocr Metab Disord. 2022 Aug;23(4):719-731. doi: 10.1007/s11154-022-09720-1. Epub 2022 Apr 5. Rev Endocr Metab Disord. 2022. PMID: 35380355 Free PMC article. Review.
-
Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions.Mol Psychiatry. 2023 Apr;28(4):1466-1479. doi: 10.1038/s41380-023-02025-y. Epub 2023 Mar 14. Mol Psychiatry. 2023. PMID: 36918706 Free PMC article. Review.
-
Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence.Int J Neuropsychopharmacol. 2019 Dec 1;22(12):754-764. doi: 10.1093/ijnp/pyz044. Int J Neuropsychopharmacol. 2019. PMID: 31420667 Free PMC article.
-
Diets Varying in Carbohydrate Content Differentially Alter Brain Activity in Homeostatic and Reward Regions in Adults.J Nutr. 2021 Aug 7;151(8):2465-2476. doi: 10.1093/jn/nxab090. J Nutr. 2021. PMID: 33852013 Free PMC article. Clinical Trial.
References
-
- Alonso‐Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, Rifkin‐Graboi A, Yannakoulia M, Rojas R, Pascual‐Leone A, Mantzoros CS (2011): Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: Relation to fasting and feeding. Am J Clin Nutr 94:377–384. - PMC - PubMed
-
- Amoroso S, Schmid‐Antomarchi H, Fosset M, Lazdunski M (1990): Glucose, sulfonylureas, and neurotransmitter release: Role of ATP‐sensitive K+ channels. Science 247:852–854. - PubMed
-
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B (1964): Activity of single neurons in the hypothalamus feeding centers: Effect of glucose. Am J Physiol 207:1146–1154. - PubMed
-
- Berthoud HR, Morrison C (2008): The brain, appetite, and obesity. Annu Rev Psychol 59:55–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

