Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance
- PMID: 27411589
- PMCID: PMC5715805
- DOI: 10.1016/j.ccell.2016.05.016
Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance
Abstract
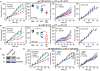
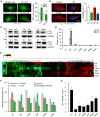
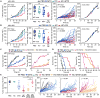
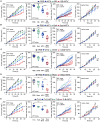
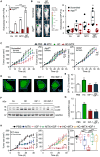
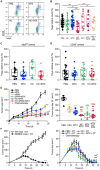
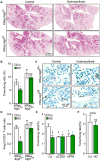
Caloric restriction mimetics (CRMs) mimic the biochemical effects of nutrient deprivation by reducing lysine acetylation of cellular proteins, thus triggering autophagy. Treatment with the CRM hydroxycitrate, an inhibitor of ATP citrate lyase, induced the depletion of regulatory T cells (which dampen anticancer immunity) from autophagy-competent, but not autophagy-deficient, mutant KRAS-induced lung cancers in mice, thereby improving anticancer immunosurveillance and reducing tumor mass. Short-term fasting or treatment with several chemically unrelated autophagy-inducing CRMs, including hydroxycitrate and spermidine, improved the inhibition of tumor growth by chemotherapy in vivo. This effect was only observed for autophagy-competent tumors, depended on the presence of T lymphocytes, and was accompanied by the depletion of regulatory T cells from the tumor bed.
Keywords: cancer; chemotherapy; immunosurveillance; regulatory T cell.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Hunger Pains: Stimulating the Appetite of the Immune System for Cancer.Cancer Cell. 2016 Jul 11;30(1):13-15. doi: 10.1016/j.ccell.2016.06.019. Cancer Cell. 2016. PMID: 27411584
References
-
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. - PubMed
-
- Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg. 2014;24:1499–1509. - PubMed
-
- Demidenko E. The assessment of tumour response to treatment. J R Stat Soc Ser C. 2006;55:365–377.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

