The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression
- PMID: 27411911
- PMCID: PMC4944446
- DOI: 10.1186/s12870-016-0845-x
The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression
Abstract
Background: Family members of sucrose non-fermenting 1-related kinase 2 (SnRK2), being plant-specific serine/threonine protein kinases, constitute the central core of abscisic acid (ABA)-dependent and ABA-independent signaling pathways, and are key regulators of abiotic stress adaptation in plants. We report here the functional characterization of SAPK9 gene, one of the 10 SnRK2s of rice, through developing gain-of-function and loss-of-function phenotypes by transgenesis.
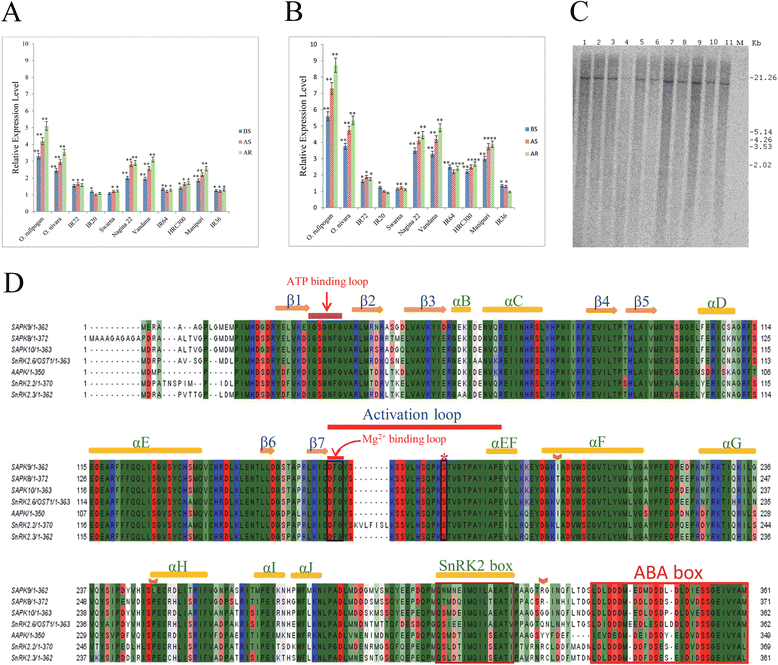
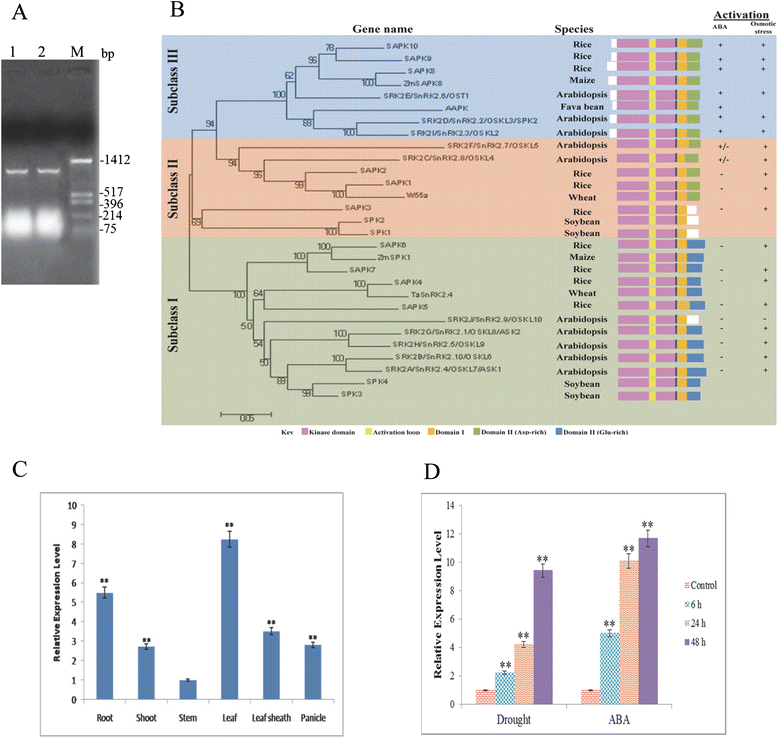
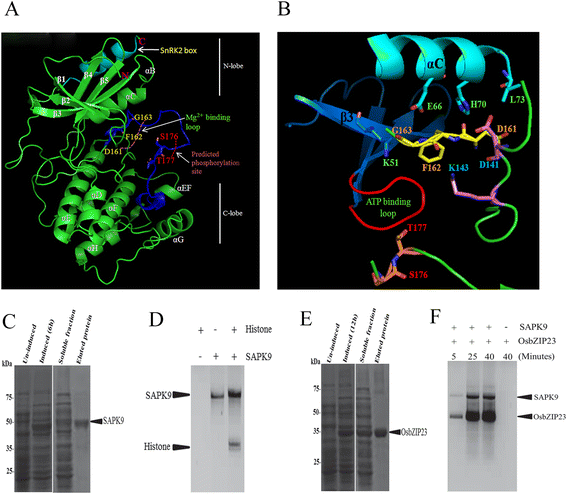
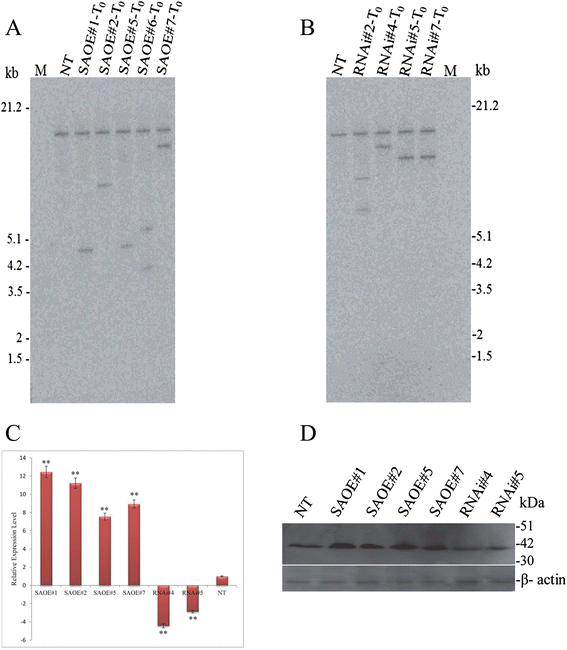
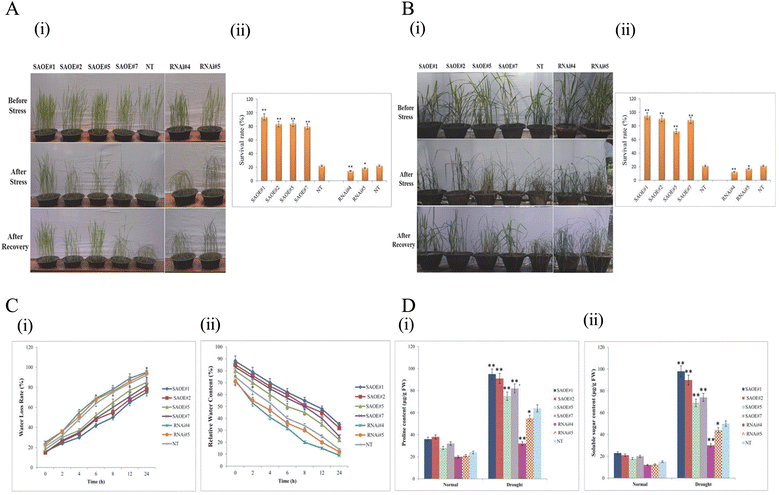
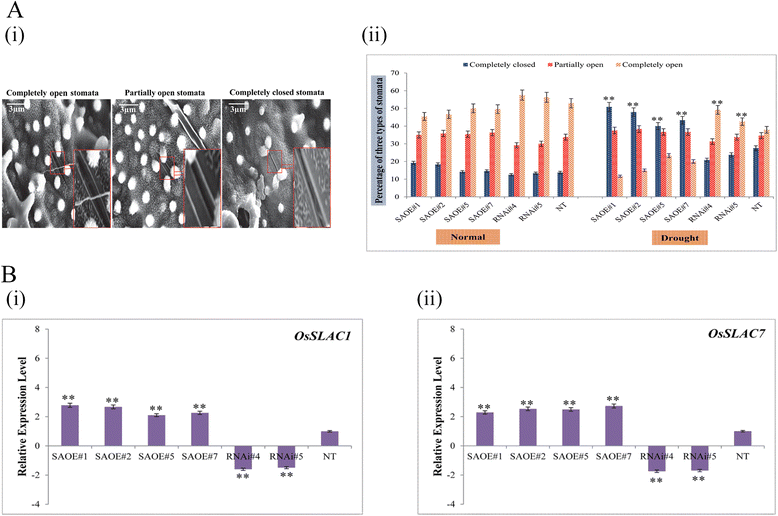
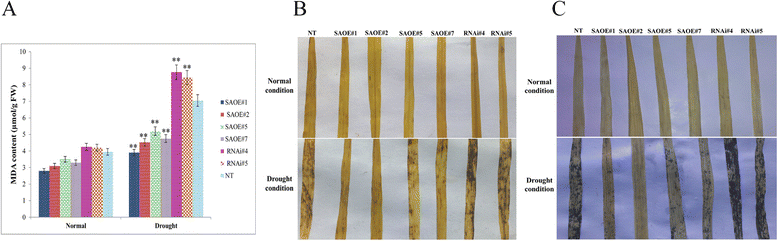
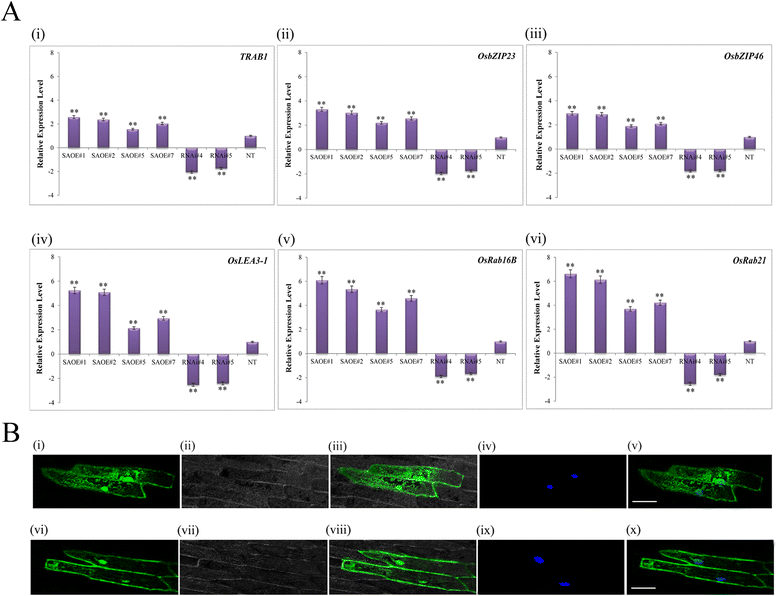
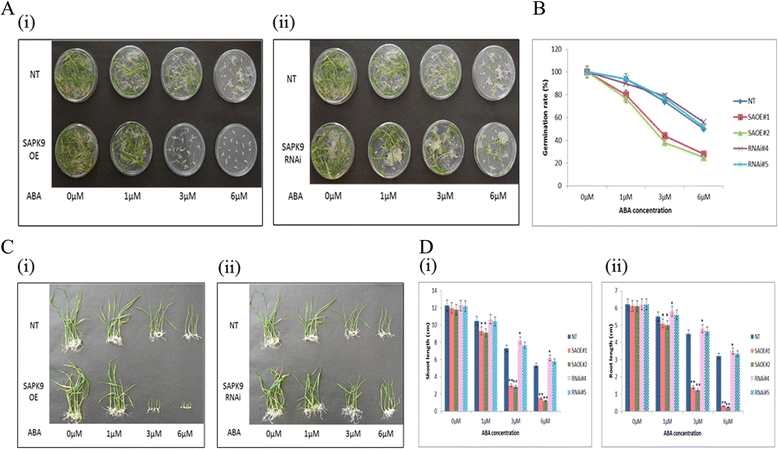
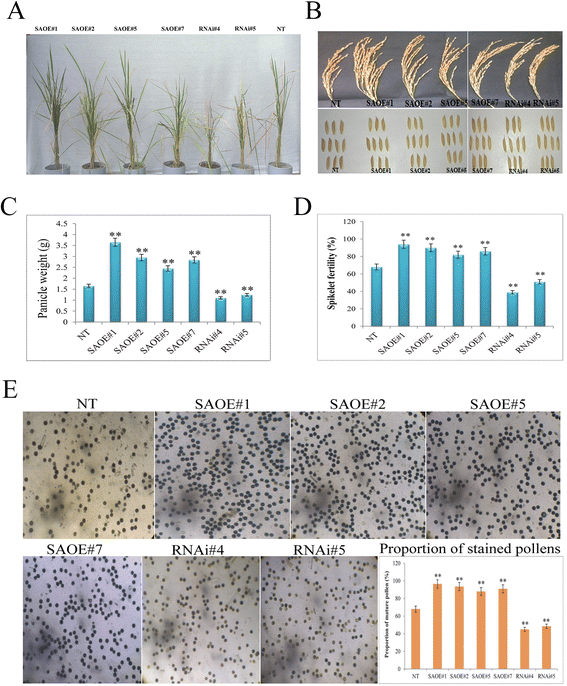
Results: The gene expression profiling revealed that the abundance of single gene-derived SAPK9 transcript was significantly higher in drought-tolerant rice genotypes than the drought-sensitive ones, and its expression was comparatively greater in reproductive stage than the vegetative stage. The highest expression of SAPK9 gene in drought-tolerant Oryza rufipogon prompted us to clone and characterise the CDS of this allele in details. The SAPK9 transcript expression was found to be highest in leaf and upregulated during drought stress and ABA treatment. In silico homology modelling of SAPK9 with Arabidopsis OST1 protein showed the bilobal kinase fold structure of SAPK9, which upon bacterial expression was able to phosphorylate itself, histone III and OsbZIP23 as substrates in vitro. Transgenic overexpression (OE) of SAPK9 CDS from O. rufipogon in a drought-sensitive indica rice genotype exhibited significantly improved drought tolerance in comparison to transgenic silencing (RNAi) lines and non-transgenic (NT) plants. In contrast to RNAi and NT plants, the enhanced drought tolerance of OE lines was concurrently supported by the upgraded physiological indices with respect to water retention capacity, soluble sugar and proline content, stomatal closure, membrane stability, and cellular detoxification. Upregulated transcript expressions of six ABA-dependent stress-responsive genes and increased sensitivity to exogenous ABA of OE lines indicate that the SAPK9 is a positive regulator of ABA-mediated stress signaling pathways in rice. The yield-related traits of OE lines were augmented significantly, which resulted from the highest percentage of fertile pollens in OE lines when compared with RNAi and NT plants.
Conclusion: The present study establishes the functional role of SAPK9 as transactivating kinase and potential transcriptional activator in drought stress adaptation of rice plant. The SAPK9 gene has potential usefulness in transgenic breeding for improving drought tolerance and grain yield in crop plants.
Keywords: Abscisic acid (ABA); Drought tolerance; Gene silencing; Grain yield; Osmotic potential; Overexpression; Rice crop; SAPK9; Stomatal closure; Stress-responsive gene; Sucrose non-fermenting 1-related kinase 2 (SnRK2).
Figures
Similar articles
-
Enhanced Gene Expression Rather than Natural Polymorphism in Coding Sequence of the OsbZIP23 Determines Drought Tolerance and Yield Improvement in Rice Genotypes.PLoS One. 2016 Mar 9;11(3):e0150763. doi: 10.1371/journal.pone.0150763. eCollection 2016. PLoS One. 2016. PMID: 26959651 Free PMC article.
-
OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2 O2 signalling in rice.Plant Biotechnol J. 2017 Feb;15(2):183-196. doi: 10.1111/pbi.12601. Epub 2016 Nov 11. Plant Biotechnol J. 2017. PMID: 27420922 Free PMC article.
-
Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance.BMC Plant Biol. 2016 Sep 20;16(1):203. doi: 10.1186/s12870-016-0897-y. BMC Plant Biol. 2016. PMID: 27646344 Free PMC article.
-
Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants.Physiol Plant. 2013 Jan;147(1):15-27. doi: 10.1111/j.1399-3054.2012.01635.x. Epub 2012 May 16. Physiol Plant. 2013. PMID: 22519646 Review.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
Cited by
-
A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice.Plant J. 2019 Jun;98(5):884-897. doi: 10.1111/tpj.14288. Epub 2019 Mar 18. Plant J. 2019. PMID: 30771248 Free PMC article.
-
An SNP based genotyping assay for genes associated with drought tolerance in bread wheat.Mol Biol Rep. 2024 Apr 18;51(1):527. doi: 10.1007/s11033-024-09481-x. Mol Biol Rep. 2024. PMID: 38637351
-
Genomic Characterization and Expression Analysis of the SnRK Family Genes in Dendrobium officinale Kimura et Migo (Orchidaceae).Plants (Basel). 2021 Mar 3;10(3):479. doi: 10.3390/plants10030479. Plants (Basel). 2021. PMID: 33802577 Free PMC article.
-
Physiological and transcriptome analyses for assessing the effects of exogenous uniconazole on drought tolerance in hemp (Cannabis sativa L.).Sci Rep. 2021 Jul 14;11(1):14476. doi: 10.1038/s41598-021-93820-6. Sci Rep. 2021. PMID: 34262091 Free PMC article.
-
Transcriptomic and Metabolomic Insights into ABA-Related Genes in Cerasus humilis under Drought Stress.Int J Mol Sci. 2024 Jul 11;25(14):7635. doi: 10.3390/ijms25147635. Int J Mol Sci. 2024. PMID: 39062878 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

