Antagonizing Retinoic Acid Receptors Increases Myeloid Cell Production by Cultured Human Hematopoietic Stem Cells
- PMID: 27412076
- PMCID: PMC5274652
- DOI: 10.1007/s00005-016-0411-0
Antagonizing Retinoic Acid Receptors Increases Myeloid Cell Production by Cultured Human Hematopoietic Stem Cells
Abstract
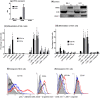
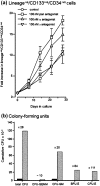
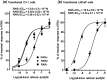
Activities of the retinoic acid receptor (RAR)α and RARγ are important to hematopoiesis. Here, we have investigated the effects of receptor selective agonists and antagonists on the primitive human hematopoietic cell lines KG1 and NB-4 and purified normal human hematopoietic stem cells (HSCs). Agonizing RARα (by AGN195183) was effective in driving neutrophil differentiation of NB-4 cells and this agonist synergized with a low amount (10 nM) of 1α,25-dihydroxyvitamin D3 to drive monocyte differentiation of NB-4 and KG1 cells. Treatment of cultures of human HSCs (supplemented with stem cell factor ± interleukin 3) with an antagonist of all RARs (AGN194310) or of RARα (AGN196996) prolonged the lifespan of cultures, up to 55 days, and increased the production of neutrophils and monocytes. Slowing down of cell differentiation was not observed, and instead, hematopoietic stem and progenitor cells had expanded in number. Antagonism of RARγ (by AGN205728) did not affect cultures of HSCs. Studies of CV-1 and LNCaP cells transfected with RAR expression vectors and a reporter vector revealed that RARγ and RARβ are activated by sub-nM all-trans retinoic acid (EC50-0.3 nM): ~50-fold more is required for activation of RARα (EC50-16 nM). These findings further support the notion that the balance of expression and activity of RARα and RARγ are important to hematopoietic stem and progenitor cell expansion and differentiation.
Keywords: Agonist; All-trans retinoic acid; Antagonist; Hematopoiesis; Monocytes; Neutrophils; Retinoic acid receptor.
Conflict of interest statement
The authors declare that there are no conflicts of interest Human CD34+ve cells were purified from the blood of normal human adult donors and post-mobilization of stem cells to the blood. Ethics approval for the use of adult human blood-mobilized stem cells (CD34+vehuHSC) was from the West Midlands Research Ethics Committee. Informed consent was obtained by the regional National Blood Service Stem Cell Laboratory in Birmingham.
Figures

Similar articles
-
Retinoic acid receptor γ is a therapeutically targetable driver of growth and survival in prostate cancer.Cancer Rep (Hoboken). 2020 Dec;3(6):e1284. doi: 10.1002/cnr2.1284. Epub 2020 Sep 3. Cancer Rep (Hoboken). 2020. PMID: 32881426 Free PMC article.
-
RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation.J Exp Med. 2006 May 15;203(5):1283-93. doi: 10.1084/jem.20052105. Epub 2006 May 8. J Exp Med. 2006. PMID: 16682494 Free PMC article.
-
Antagonists of retinoic acid receptors (RARs) are potent growth inhibitors of prostate carcinoma cells.Br J Cancer. 2001 Aug 3;85(3):453-62. doi: 10.1054/bjoc.2001.1939. Br J Cancer. 2001. PMID: 11487280 Free PMC article.
-
Molecular Interactions of Selective Agonists and Antagonists with the Retinoic Acid Receptor γ.Int J Mol Sci. 2024 Jun 14;25(12):6568. doi: 10.3390/ijms25126568. Int J Mol Sci. 2024. PMID: 38928275 Free PMC article. Review.
-
Function of RARalpha during the maturation of neutrophils.Oncogene. 2001 Oct 29;20(49):7178-85. doi: 10.1038/sj.onc.1204757. Oncogene. 2001. PMID: 11704846 Review.
Cited by
-
Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media.Int J Stem Cells. 2020 Nov 30;13(3):326-334. doi: 10.15283/ijsc19157. Int J Stem Cells. 2020. PMID: 32840223 Free PMC article. Review.
-
Targeting Androgen, Thyroid Hormone, and Vitamin A and D Receptors to Treat Prostate Cancer.Int J Mol Sci. 2024 Aug 26;25(17):9245. doi: 10.3390/ijms25179245. Int J Mol Sci. 2024. PMID: 39273194 Free PMC article. Review.
-
Retinoic acid receptor γ is a therapeutically targetable driver of growth and survival in prostate cancer.Cancer Rep (Hoboken). 2020 Dec;3(6):e1284. doi: 10.1002/cnr2.1284. Epub 2020 Sep 3. Cancer Rep (Hoboken). 2020. PMID: 32881426 Free PMC article.
-
The Emerging Oncogenic Role of RARγ: From Stem Cell Regulation to a Potential Cancer Therapy.Int J Mol Sci. 2025 May 3;26(9):4357. doi: 10.3390/ijms26094357. Int J Mol Sci. 2025. PMID: 40362593 Free PMC article. Review.
-
Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells.Int J Mol Sci. 2023 Jan 25;24(3):2373. doi: 10.3390/ijms24032373. Int J Mol Sci. 2023. PMID: 36768694 Free PMC article. Review.
References
-
- Allegretto E, McClurg MR, Lazarchik SB, et al. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast. Correlation with hormone binding and effects of metabolism. J Biol Chem. 1993;268:26625–26633. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

