Differential cytotoxicity induced by the Titanium(IV)Salan complex Tc52 in G2-phase independent of DNA damage
- PMID: 27412346
- PMCID: PMC4944496
- DOI: 10.1186/s12885-016-2538-0
Differential cytotoxicity induced by the Titanium(IV)Salan complex Tc52 in G2-phase independent of DNA damage
Abstract
Background: Chemotherapy is one of the major treatment modalities for cancer. Metal-based compounds such as derivatives of cisplatin are in the front line of therapy against a subset of cancers, but their use is restricted by severe side-effects and the induction of resistance in treated tumors. Subsequent research focused on development of cytotoxic metal-complexes without cross-resistance to cisplatin and reduced side-effects. This led to the discovery of first-generation titanium(IV)salan complexes, which reached clinical trials but lacked efficacy. New-generation titanium (IV)salan-complexes show promising anti-tumor activity in mice, but their molecular mechanism of cytotoxicity is completely unknown.
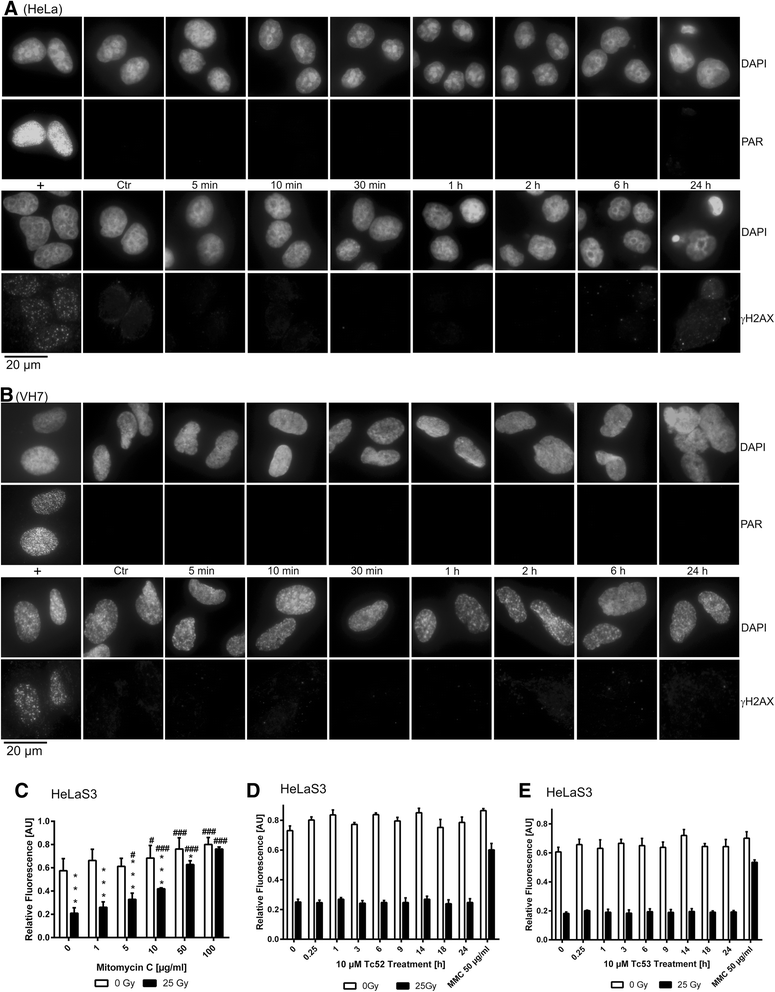
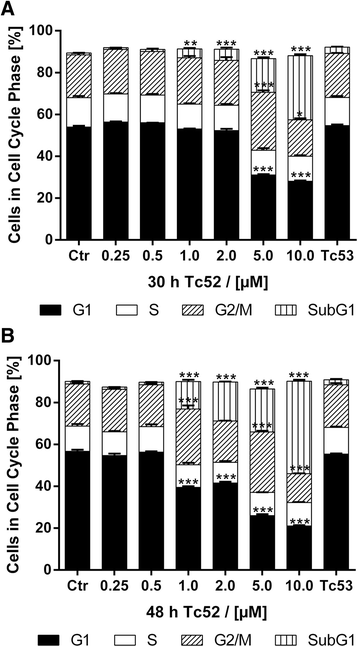
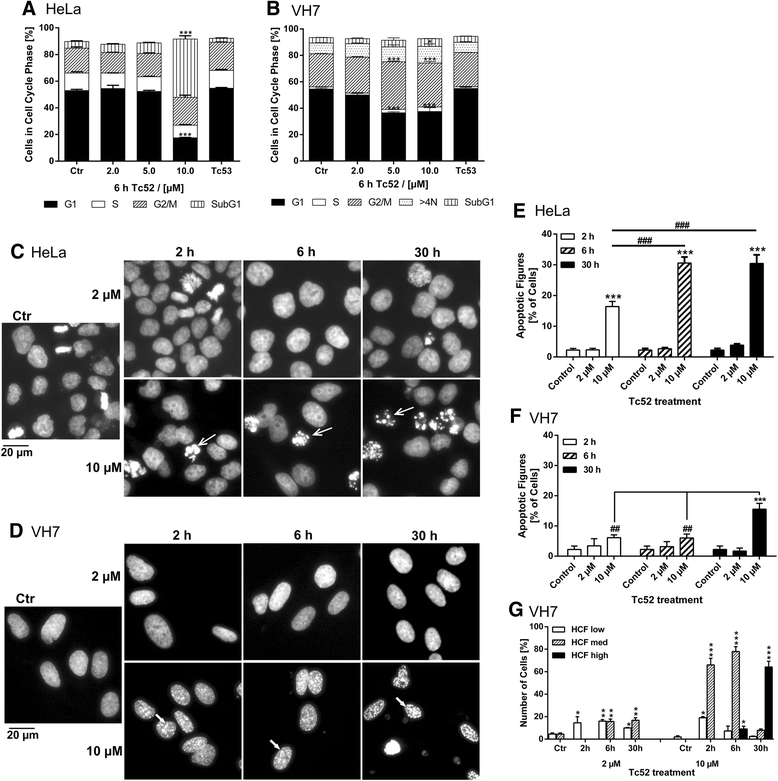
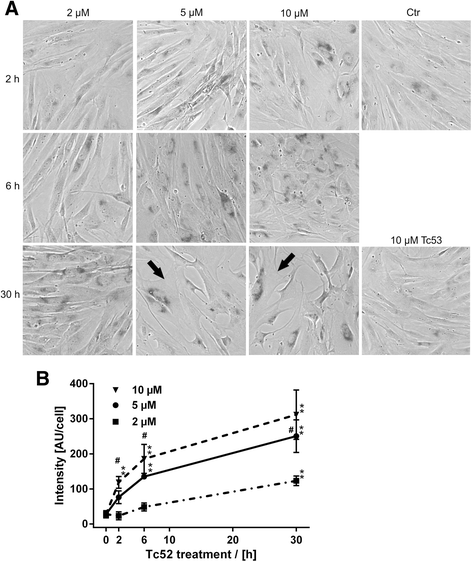
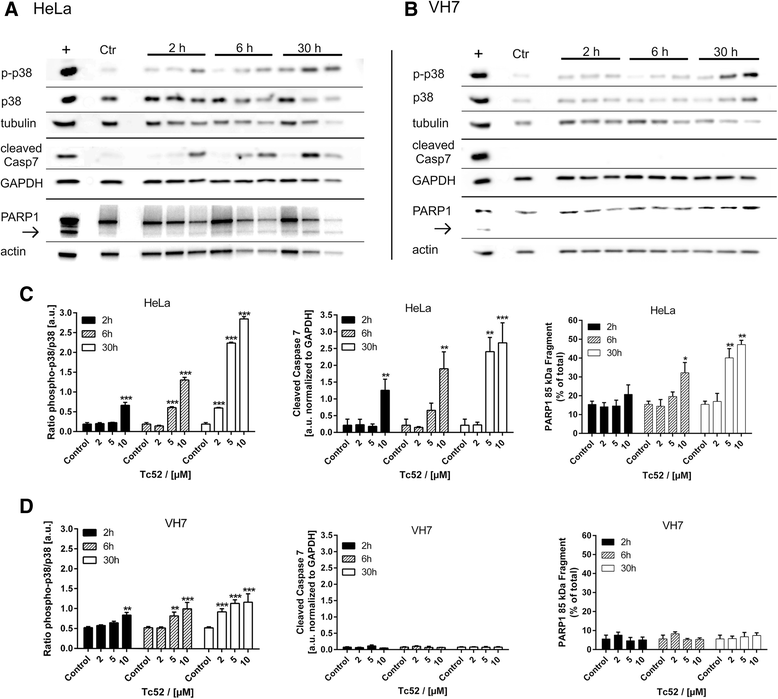
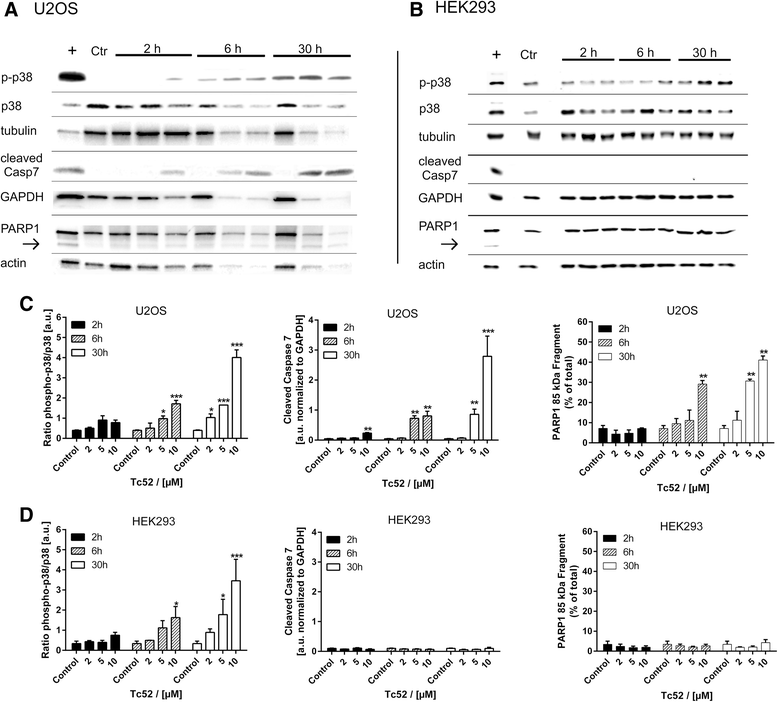
Methods: Four different human cell lines were analyzed in their responses to a toxic (Tc52) and a structurally highly related but non-toxic (Tc53) titanium(IV)salan complex. Viability assays were used to reveal a suitable treatment range, flow-cytometry analysis was performed to monitor the impact of dosage and treatment time on cell-cycle distribution and cell death. Potential DNA strand break induction and crosslinking was investigated by immunostaining of damage markers as well as automated fluorometric analysis of DNA unwinding. Changes in nuclear morphology were analyzed by DAPI staining. Acidic beta-galactosidase activity together with morphological changes was monitored to detect cellular senescence. Western blotting was used to analyze induction of pro-apoptotic markers such as activated caspase7 and cleavage of PARP1, and general stress kinase p38.
Results: Here we show that the titanium(IV)salan Tc52 is effective in inducing cell death in the lower micromolar range. Surprisingly, Tc52 does not target DNA contrary to expectations deduced from the reported activity of other titanium complexes. Instead, Tc52 application interferes with progression from G2-phase into mitosis and induces apoptotic cell death in tested tumor cells. Contrarily, human fibroblasts undergo senescence in a time and dose-dependent manner. As deduced from fluorescence studies, the potential cellular target seems to be the cytoskeleton.
Conclusions: In summary, we could demonstrate in four different human cell lines that tumor cells were specifically killed without induction of major cytotoxicity in non-tumorigenic cells. Absence of DNA damaging activity and the cell-cycle block in G2 instead of mitosis makes Tc52 an attractive compound for further investigations in cancer treatment.
Keywords: Apoptosis; Cell-cycle; Senescence; Titanium(IV)salan complex; Tumorigenicity.
Figures

Similar articles
-
Cytotoxic salan-titanium(IV) complexes: high activity toward a range of sensitive and drug-resistant cell lines, and mechanistic insights.ChemMedChem. 2012 Apr;7(4):703-8. doi: 10.1002/cmdc.201100593. Epub 2012 Jan 20. ChemMedChem. 2012. PMID: 22262543
-
Cytotoxic titanium salan complexes: surprising interaction of salan and alkoxy ligands.Chemistry. 2010 Mar 1;16(9):2775-89. doi: 10.1002/chem.200902312. Chemistry. 2010. PMID: 20104550
-
High antitumor activity of highly resistant salan-titanium(IV) complexes in nanoparticles: an identified active species.Angew Chem Int Ed Engl. 2012 Oct 15;51(42):10515-7. doi: 10.1002/anie.201205973. Epub 2012 Sep 7. Angew Chem Int Ed Engl. 2012. PMID: 22961758
-
Coordination Complexes of Titanium(IV) for Anticancer Therapy.Met Ions Life Sci. 2018 Feb 5;18:/books/9783110470734/9783110470734-014/9783110470734-014.xml. doi: 10.1515/9783110470734-014. Met Ions Life Sci. 2018. PMID: 29394027 Review.
-
Mechanisms of cytotoxicity of anticancer titanocenes.Anticancer Agents Med Chem. 2010 May;10(4):302-11. doi: 10.2174/187152010791162261. Anticancer Agents Med Chem. 2010. PMID: 20380637 Review.
Cited by
-
Probing the Mechanism of Action of Bis(phenolato) Amine (ONO Donor Set) Titanium(IV) Anticancer Agents.J Med Chem. 2024 Feb 22;67(4):2732-2744. doi: 10.1021/acs.jmedchem.3c01874. Epub 2024 Feb 8. J Med Chem. 2024. PMID: 38331433 Free PMC article.
-
The relationship between vacuolation and initiation of PCD in rice (Oryza sativa) aleurone cells.Sci Rep. 2017 Jan 24;7:41245. doi: 10.1038/srep41245. Sci Rep. 2017. PMID: 28117452 Free PMC article.
-
The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion.J Cell Mol Med. 2018 Dec;22(12):6039-6054. doi: 10.1111/jcmm.13879. Epub 2018 Sep 14. J Cell Mol Med. 2018. PMID: 30596396 Free PMC article.
-
Exploring the Potential of Metal-Based Candidate Drugs as Modulators of the Cytoskeleton.Chembiochem. 2023 Sep 1;24(17):e202300178. doi: 10.1002/cbic.202300178. Epub 2023 Aug 1. Chembiochem. 2023. PMID: 37345897 Free PMC article. Review.
References
-
- Lohia S, Rajapurkar M, Nguyen SA, Sharma AK, Gillespie MB, Day TA. A Comparison of Outcomes Using Intensity-Modulated Radiation Therapy and 3-Dimensional Conformal Radiation Therapy in Treatment of Oropharyngeal Cancer. JAMA Otolaryngol Head Neck Surg. 2014. - PubMed
-
- Mima M, Demizu Y, Jin D, Hashimoto N, Takagi M, Terashima K, Fujii O, Niwa Y, Akagi T, Daimon T, et al. Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. Br J Radiol. 2014;87(1033):20130512. doi: 10.1259/bjr.20130512. - DOI - PMC - PubMed
-
- Puisset F, Schmitt A, Chatelut E. Standardization of chemotherapy and individual dosing of platinum compounds. Anticancer Res. 2014;34(1):465–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

