Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period
- PMID: 27412362
- PMCID: PMC6461153
- DOI: 10.1002/14651858.CD011352.pub2
Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period
Update in
-
Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period.Cochrane Database Syst Rev. 2021 Jan 11;1(1):CD011352. doi: 10.1002/14651858.CD011352.pub3. Cochrane Database Syst Rev. 2021. PMID: 33427305 Free PMC article.
Abstract
Background: Many women experience perineal pain after childbirth, especially after having sustained perineal trauma. Perineal pain-management strategies are thus an important part of postnatal care. Non-steroidal anti-inflammatory drugs (NSAIDs) are a commonly used type of medication in the management of postpartum pain and their effectiveness and safety should be assessed.
Objectives: To determine the effectiveness of a single dose of an oral NSAID for relief of acute perineal pain in the early postpartum period.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2016), OpenSIGLE, ProQuest Dissertations and Theses, the ISRCTN Registry and ClinicalTrials.gov (31 March 2016). We also reviewed reference lists of retrieved papers and contacted experts in the field.
Selection criteria: Randomised controlled trials (RCTs) assessing a single dose of a NSAID versus a single dose of placebo, paracetamol or another NSAID for women with perineal pain in the early postpartum period. Quasi-RCTs and cross-over trials were excluded.
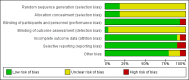
Data collection and analysis: Two review authors (FW and VS) independently assessed all identified papers for inclusion and risk of bias. Any discrepancies were resolved through discussion and consensus. Data extraction, including calculations of pain relief scores, was also conducted independently by two review authors and checked for accuracy.
Main results: We included 28 studies that examined 13 different NSAIDs and involved 4181 women (none of whom were breastfeeding). Studies were published between 1967 and 2013, with the majority published in the 1980s. Of the 4181 women involved in the studies, 2642 received a NSAID and 1539 received placebo or paracetamol. Risk of bias was generally unclear due to poor reporting, but in most studies the participants and personnel were blinded, outcome data were complete and the outcomes that were specified in the methods section were reported.None of the included studies reported on any of this review's secondary outcomes: prolonged hospitalisation or re-hospitalisation due to perineal pain; breastfeeding (fully or mixed) at discharge; breastfeeding (fully or mixed) at six weeks; perineal pain at six weeks; maternal views; postpartum depression; instrumental measures of disability due to perineal pain. NSAID versus placeboCompared to women who received a placebo, more women who received a single dose NSAID achieved adequate pain relief at four hours (risk ratio (RR) 1.91, 95% confidence interval (CI) 1.64 to 2.23, 10 studies, 1573 participants (low-quality evidence)) and adequate pain relief at six hours (RR 1.92, 95% CI 1.69 to 2.17, 17 studies, 2079 participants (very low-quality evidence)). Women who received a NSAID were also less likely to need additional analgesia compared to women who received placebo at four hours (RR 0.39, 95% CI 0.26 to 0.58, four studies, 486 participants (low-quality evidence)) and at six hours after initial administration (RR 0.32, 95% CI 0.26 to 0.40, 10 studies, 1012 participants (low-quality evidence)). Fourteen maternal adverse effects were reported in the NSAID group (drowsiness (5), abdominal discomfort (2), weakness (1), dizziness (2), headache (2), moderate epigastralgia (1), not specified (1)) and eight in the placebo group (drowsiness (2), light headed (1), nausea (1), backache (1), dizziness (1), epigastric pain (1), not specified (1)), although not all studies assessed adverse effects. There was no difference in overall maternal adverse effects between NSAIDs and placebo at six hours post-administration (RR 1.38, 95% CI 0.71 to 2.70, 13 studies, 1388 participants (very low-quality evidence)). One small study (with two treatment arms) assessed maternal adverse effects at four hours post-administration, but there were no maternal adverse effects observed (one study, 90 participants (low-quality evidence)). Neonatal adverse effects were not assessed in any of the included studies. NSAID versus paracetamolNSAIDs versus paracetamol were also more effective for adequate pain relief at four hours (RR 1.54, 95% CI 1.07 to 2.22, three studies, 342 participants) but not at six hours post-administration. There was no difference in the need for additional analgesia between the two groups at four hours (RR 0.55, 95% CI 0.27 to 1.13, one study, 73 participants), but women in the NSAID group were less likely to need any additional analgesia at six hours (RR 0.28, 95% CI 0.12 to 0.67, one study, 59 participants). No maternal adverse effects were reported four hours after drug administration (one study). Six hours post-administration, there was no difference between the groups in the number of maternal adverse effects (RR 0.74, 95% CI 0.27 to 2.08, three studies, 300 participants), with one case of pruritis in the NSAID group and one case of sleepiness in the paracetamol group. Neonatal adverse effects were not assessed in any of the included studies.Comparisons of different NSAIDs and different doses of the same NSAID did not demonstrate any differences in their effectiveness on any of the primary outcome measures; however, few data were available on some NSAIDs.
Authors' conclusions: In women who are not breastfeeding and who sustained perineal trauma, NSAIDs (compared to placebo) provide greater pain relief for acute postpartum perineal pain and fewer women need additional analgesia when treated with a NSAID. However, the risk of bias was unclear for many of the included studies, adverse effects were often not assessed and breastfeeding women were not included in the studies. The overall quality of the evidence (GRADE) was low with the evidence for all outcomes rated as low or very low. The main reasons for downgrading were inclusion of studies with high risk of bias and inconsistency of findings of individual studies.NSAIDs also appear to be more effective in providing relief for perineal pain than paracetamol, but few studies were included in this analysis.Future studies should examine NSAIDs' adverse effects profile including neonatal adverse effects and the compatibility of NSAIDs with breastfeeding, and assess other important secondary outcomes of this review. Moreover, studies mostly included women who had episiotomies. Future research should consider women with and without perineal trauma, including perineal tears. High-quality studies should be conducted to further assess the efficacy of NSAIDs versus paracetamol and the efficacy of multimodal treatments.
Conflict of interest statement
Francesca Wuytack: has received a Cochrane Fellowship, awarded by the Health Research Board Ireland, to support the conduct of this review.
Valerie Smith has acted in the capacity of Ms Wuytack's supervisor and PI on the Cochrane HRB Fellowship grant application. The awarded grant is not salary or such support, rather support for Ms Wuytack to attend Cochrane training workshops and other necessary consumables.
Brian J Cleary's institution has received €500from the State Claims Agency for a lecture that he gave about the historical context of the development and marketing of thalidomide in Ireland to a mediation process between the Irish state and survivors of the Thalidomide disaster. A sum of €1500 was also paid to his institution (by Ferring Pharmaceuticals) for training provided to private sector pharmacies and an infertility clinic on the use of a specialised drug delivery system used to delivery an infertility treatment from Ferring (Lutrelef). Brian's brother works for a drug company that sells analgesic products (Grunenthal). However Brian's institution does not currently use their products for the management of peripartum pain and their products are irrelevant to the content of this review. Brian has been publicly critical of Grunenthal and their historical actions in the context of the thalidomide disaster.
Figures


























Similar articles
-
Aspirin (single dose) for perineal pain in the early postpartum period.Cochrane Database Syst Rev. 2017 Feb 9;2(2):CD012129. doi: 10.1002/14651858.CD012129.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jul 24;7:CD012129. doi: 10.1002/14651858.CD012129.pub3. PMID: 28181214 Free PMC article. Updated.
-
Oral non-steroidal anti-inflammatory drugs (single dose) for perineal pain in the early postpartum period.Cochrane Database Syst Rev. 2021 Jan 11;1(1):CD011352. doi: 10.1002/14651858.CD011352.pub3. Cochrane Database Syst Rev. 2021. PMID: 33427305 Free PMC article.
-
Oral analgesia for relieving post-caesarean pain.Cochrane Database Syst Rev. 2015 Mar 29;2015(3):CD010450. doi: 10.1002/14651858.CD010450.pub2. Cochrane Database Syst Rev. 2015. PMID: 25821010 Free PMC article.
-
Interventions for preventing postpartum constipation.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011625. doi: 10.1002/14651858.CD011625.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Aug 5;8:CD011625. doi: 10.1002/14651858.CD011625.pub3. PMID: 26387487 Free PMC article. Updated.
-
Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury.Cochrane Database Syst Rev. 2015 Jul 1;(7):CD007789. doi: 10.1002/14651858.CD007789.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Aug 12;8:CD007789. doi: 10.1002/14651858.CD007789.pub3. PMID: 26130144 Updated.
Cited by
-
Aspirin (single dose) for perineal pain in the early postpartum period.Cochrane Database Syst Rev. 2020 Jul 24;7(7):CD012129. doi: 10.1002/14651858.CD012129.pub3. Cochrane Database Syst Rev. 2020. PMID: 32702783 Free PMC article.
-
Guizhi Fuling Capsule Exhibits Antidysmenorrhea Activity by Inhibition of Cyclooxygenase Activity.Evid Based Complement Alternat Med. 2020 May 23;2020:8607931. doi: 10.1155/2020/8607931. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32595743 Free PMC article.
-
Antenatal maternal education for improving postnatal perineal healing for women who have birthed in a hospital setting.Cochrane Database Syst Rev. 2017 Dec 4;12(12):CD012258. doi: 10.1002/14651858.CD012258.pub2. Cochrane Database Syst Rev. 2017. PMID: 29205275 Free PMC article.
-
Oral tramadol versus oral celecoxib for analgesia after mediolateral episiotomy repair in obese primigravidae: a randomized controlled trial.Int Urogynecol J. 2021 Sep;32(9):2465-2472. doi: 10.1007/s00192-020-04411-4. Epub 2020 Jul 20. Int Urogynecol J. 2021. PMID: 32691120 Clinical Trial.
-
Local cooling for relieving pain from perineal trauma sustained during childbirth.Cochrane Database Syst Rev. 2020 Oct 9;10(10):CD006304. doi: 10.1002/14651858.CD006304.pub4. Cochrane Database Syst Rev. 2020. PMID: 33034900 Free PMC article.
References
References to studies included in this review
-
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, et al. Analgesic effect of ibuprofen in pain after episiotomy [Effets antalgiques de l'ibuprofene dans les douleurs apres episiotomie]. Annales Francaises d Anesthesie et de Reanimation 1992;11(1):22‐6. [] - PubMed
-
3226420
-
- Bloomfield SS, Gaffney TE, Howett M. Comparative analgesic efficacy of chlorphenesin carbamate and acetylsalicylic acid after episiotomy. Anesthesia and Analgesia 1967;46:515‐20. [] - PubMed
-
3226422
-
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15:565‐70. [] - PubMed
-
3226424
-
- Daftary SN, Mehta AC, Nanavati M. A controlled comparison of dipyrone and paracetamol in post‐episiotomy pain. Current Medical Research and Opinion 1980;6:614‐8. [] - PubMed
-
3226426
-
- Vroey P. A double‐blind comparison of diflunisal and aspirin in the treatment of post‐operative pain after episiotomy. Current Medical Research and Opinion 1978;5:544‐7. [] - PubMed
- Vroey P. The treatment of postoperative pain with a single dose of diflunisal. Clinical Therapeutics 1977;1(Suppl A):30‐3. []
-
3226428
References to studies excluded from this review
-
- Abedzadeh M, Sadat Z, Saberi F. The efficacy of 2% lidocaine gel versus diclofenac suppository in pain reliving after episiotomy. Koomesh 2009;10(4):301‐5. []
-
3226480
-
- Akil A, Api O, Bektas Y, Yilmaz AO, Yalti S, Unal O. Paracetamol vs dexketoprofen for perineal pain relief after episiotomy or perineal tear. Journal of Obstetrics and Gynaecology 2014;34(1):25‐8. [] - PubMed
-
3226482
-
- Altungul AC, Sapmaz E, Kale A. Comparison of diclofenac sodium with indomethacin suppositories for mediolateral episiotomies. Clinical and Experimental Obstetrics and Gynecology 2012;39(1):112‐4. [] - PubMed
-
3226484
-
- Bettigole JB. A double‐blind comparison of placebo, codeine, and fenoprofen in patients with postpartum pain. Current Therapeutic Research 1981;29:778‐84. []
-
3226486
-
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post‐episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34‐8. [] - PubMed
-
3226488
Additional references
-
- Abou‐Saleh MT, Ghubash R. The prevalence of early postpartum psychiatric morbidity in Dubai: transcultural perspective. Acta Psychiatrica Scandinavica 1997;95:428‐32. - PubMed
-
- Andrews V, Thakar R, Sultan AH, Jones PW. Evaluation of postpartum perineal pain and dyspareunia‐‐a prospective study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;137:152‐6. - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

