Downregulation of Heme Oxygenase 1 (HO-1) Activity in Hematopoietic Cells Enhances Their Engraftment After Transplantation
- PMID: 27412411
- PMCID: PMC5538806
- DOI: 10.3727/096368915X688957
Downregulation of Heme Oxygenase 1 (HO-1) Activity in Hematopoietic Cells Enhances Their Engraftment After Transplantation
Abstract
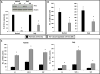
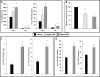
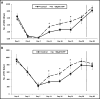
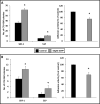
Heme oxygenase 1 (HO-1) is an inducible stress-response enzyme that not only catalyzes the degradation of heme (e.g., released from erythrocytes) but also has an important function in various physiological and pathophysiological states associated with cellular stress, such as ischemic/reperfusion injury. HO-1 has a well-documented anti-inflammatory potential, and HO-1 has been reported to have a negative effect on adhesion and migration of neutrophils in acute inflammation in a model of peritonitis. This finding is supported by our recent observation that hematopoietic stem progenitor cells (HSPCs) from HO-1 KO mice are easy mobilizers, since they respond better to peripheral blood chemotactic gradients than wild-type littermates. Based on these findings, we hypothesized that transient inhibition of HO-1 by nontoxic small-molecule inhibitors would enhance migration of HSPCs in response to bone marrow chemoattractants and thereby facilitate their homing. To directly address this issue, we generated several human hematopoietic cell lines in which HO-1 was upregulated or downregulated. We also exposed murine and human BM-derived cells to small-molecule activators and inhibitors of HO-1. Our results indicate that HO-1 is an inhibitor of hematopoietic cell migration in response to crucial BM homing chemoattractants such as stromal-derived factor 1 (SDF-1) and sphingosine-1-phosphate (S1P). Most importantly, our in vitro and in vivo animal experiments demonstrate for the first time that transiently inhibiting HO-1 activity in HSPCs by small-molecule inhibitors improves HSPC engraftment. We propose that this simple and inexpensive strategy could be employed in the clinical setting to improve engraftment of HSPCs, particularly in those situations in which the number of HSPCs available for transplant is limited (e.g., when transplanting umbilical cord blood).
Conflict of interest statement
None.
Figures

Similar articles
-
The Involvment of Hematopoietic-Specific PLC -β2 in Homing and Engraftment of Hematopoietic Stem/Progenitor Cells.Stem Cell Rev Rep. 2016 Dec;12(6):613-620. doi: 10.1007/s12015-016-9689-x. Stem Cell Rev Rep. 2016. PMID: 27704316 Free PMC article.
-
Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells.Stem Cell Rev Rep. 2015 Feb;11(1):110-8. doi: 10.1007/s12015-014-9547-7. Stem Cell Rev Rep. 2015. PMID: 25086571 Free PMC article.
-
Evidence for the involvement of sphingosine-1-phosphate in the homing and engraftment of hematopoietic stem cells to bone marrow.Oncotarget. 2015 Aug 7;6(22):18819-28. doi: 10.18632/oncotarget.4710. Oncotarget. 2015. PMID: 26299919 Free PMC article.
-
Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells.Stem Cell Rev Rep. 2016 Feb;12(1):121-8. doi: 10.1007/s12015-015-9625-5. Stem Cell Rev Rep. 2016. PMID: 26400757 Free PMC article. Review.
-
Heme Oxygenase 1 (HO-1) as an Inhibitor of Trafficking of Normal and Malignant Hematopoietic Stem Cells - Clinical and Translational Implications.Stem Cell Rev Rep. 2021 Jun;17(3):821-828. doi: 10.1007/s12015-020-10083-w. Epub 2020 Nov 16. Stem Cell Rev Rep. 2021. PMID: 33196976 Free PMC article. Review.
Cited by
-
Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts.Stem Cell Rev Rep. 2020 Oct;16(5):954-967. doi: 10.1007/s12015-020-10005-w. Stem Cell Rev Rep. 2020. PMID: 32661868 Free PMC article.
-
Pineal Gland Hormone Melatonin Inhibits Migration of Hematopoietic Stem/Progenitor Cells (HSPCs) by Downregulating Nlrp3 Inflammasome and Upregulating Heme Oxygenase-1 (HO-1) Activity.Stem Cell Rev Rep. 2024 Jan;20(1):237-246. doi: 10.1007/s12015-023-10638-7. Epub 2023 Oct 9. Stem Cell Rev Rep. 2024. PMID: 37812364
-
Extracellular Adenosine Triphosphate (eATP) and Its Metabolite, Extracellular Adenosine (eAdo), as Opposing "Yin-Yang" Regulators of Nlrp3 Inflammasome in the Trafficking of Hematopoietic Stem/Progenitor Cells.Front Immunol. 2021 Jan 29;11:603942. doi: 10.3389/fimmu.2020.603942. eCollection 2020. Front Immunol. 2021. PMID: 33584673 Free PMC article. Review.
-
Does it make sense to target one tumor cell chemotactic factor or its receptor when several chemotactic axes are involved in metastasis of the same cancer?Clin Transl Med. 2016 Dec;5(1):28. doi: 10.1186/s40169-016-0113-6. Epub 2016 Aug 10. Clin Transl Med. 2016. PMID: 27510263 Free PMC article. Review.
-
Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation.F1000Res. 2019 Oct 31;8:F1000 Faculty Rev-1833. doi: 10.12688/f1000research.20002.1. eCollection 2019. F1000Res. 2019. PMID: 31723413 Free PMC article. Review.
References
-
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–67. - PubMed
-
- Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. - PubMed
-
- Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 2000;72:408–11. - PubMed
-
- Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26:54–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

