Type 1 Diabetes Mellitus Donor Mesenchymal Stromal Cells Exhibit Comparable Potency to Healthy Controls In Vitro
- PMID: 27412884
- PMCID: PMC5070499
- DOI: 10.5966/sctm.2015-0272
Type 1 Diabetes Mellitus Donor Mesenchymal Stromal Cells Exhibit Comparable Potency to Healthy Controls In Vitro
Abstract
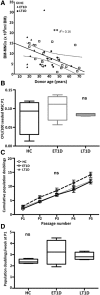
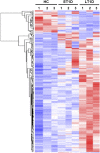
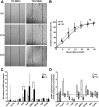
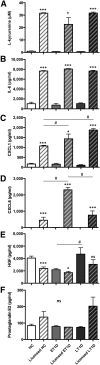
: Bone marrow mesenchymal stromal cells (BM-MSCs) have been characterized and used in many clinical studies based on their immunomodulatory and regenerative properties. We have recently reported the benefit of autologous MSC systemic therapy in the treatment of type 1 diabetes mellitus (T1D). Compared with allogeneic cells, use of autologous products reduces the risk of eliciting undesired complications in the recipient, including rejection, immunization, and transmission of viruses and prions; however, comparable potency of autologous cells is required for this treatment approach to remain feasible. To date, no analysis has been reported that phenotypically and functionally characterizes MSCs derived from newly diagnosed and late-stage T1D donors in vitro with respect to their suitability for systemic immunotherapy. In this study, we used gene array in combination with functional in vitro assays to address these questions. MSCs from T1D donors and healthy controls were expanded from BM aspirates. BM mononuclear cell counts and growth kinetics were comparable between the groups, with equivalent colony-forming unit-fibroblast capacity. Gene microarrays demonstrated differential gene expression between healthy and late-stage T1D donors in relation to cytokine secretion, immunomodulatory activity, and wound healing potential. Despite transcriptional differences, T1D MSCs did not demonstrate a significant difference from healthy controls in immunosuppressive activity, migratory capacity, or hemocompatibility. We conclude that despite differential gene expression, expanded MSCs from T1D donors are phenotypically and functionally similar to healthy control MSCs with regard to their immunomodulatory and migratory potential, indicating their suitability for use in autologous systemic therapy.
Significance: The potential for mesenchymal stromal cells (MSCs) as a cell-based therapy in the treatment of immunologic disorders has been well established. Recent studies reported the clinical potential for autologous MSCs as a systemic therapy in the treatment of type I diabetes mellitus (T1D). The current study compared the genotypic and phenotypic profiles of bone marrow-derived MSCs from T1D and healthy donors as autologous (compared with allogeneic) therapy provides distinct advantages, such as reduced risk of immune reaction and transmission of infectious agents. The findings of the current study demonstrate that despite moderate differences in T1D MSCs at the gene level, these cells can be expanded in culture to an extent corresponding to that of MSCs derived from healthy donors. No functional difference in terms of immunosuppressive activity, blood compatibility, or migratory capacity was evident between the groups. The study findings also show that autologous MSC therapy holds promise as a T1D treatment and should be evaluated further in clinical trials.
Keywords: Adult human bone marrow; Cellular therapy; Diabetes; Immunosuppression; Mesenchymal stem cells.
©AlphaMed Press.
Figures

References
-
- Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. - PubMed
-
- Mangialardi G, Oikawa A, Reni C, et al. Bone marrow microenvironment: A newly recognized target for diabetes-induced cellular damage. Endocr Metab Immune Disord Drug Targets. 2012;12:159–167. - PubMed
-
- Hematti P, Kim J, Stein AP, et al. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev (Orlando) 2013;27:21–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

