The antigenic complex in HIT binds to B cells via complement and complement receptor 2 (CD21)
- PMID: 27412887
- PMCID: PMC5054694
- DOI: 10.1182/blood-2016-04-709634
The antigenic complex in HIT binds to B cells via complement and complement receptor 2 (CD21)
Abstract
Heparin-induced thrombocytopenia is a prothrombotic disorder caused by antibodies to platelet factor 4 (PF4)/heparin complexes. The mechanism that incites such prevalent anti-PF4/heparin antibody production in more than 50% of patients exposed to heparin in some clinical settings is poorly understood. To investigate early events associated with antigen exposure, we first examined the interaction of PF4/heparin complexes with cells circulating in whole blood. In healthy donors, PF4/heparin complexes bind preferentially to B cells (>90% of B cells bind to PF4/heparin in vitro) relative to neutrophils, monocytes, or T cells. Binding of PF4 to B cells is heparin dependent, and PF4/heparin complexes are found on circulating B cells from some, but not all, patients receiving heparin. Given the high proportion of B cells that bind PF4/heparin, we investigated complement as a mechanism for noncognate antigen recognition. Complement is activated by PF4/heparin complexes, co-localizes with antigen on B cells from healthy donors, and is present on antigen-positive B cells in patients receiving heparin. Binding of PF4/heparin complexes to B cells is mediated through the interaction between complement and complement receptor 2 (CR2 [CD21]). To the best of our knowledge, these are the first studies to demonstrate complement activation by PF4/heparin complexes, opsonization of PF4/heparin to B cells via CD21, and the presence of complement activation fragments on circulating B cells in some patients receiving heparin. Given the critical contribution of complement to humoral immunity, our observations provide new mechanistic insights into the immunogenicity of PF4/heparin complexes.
© 2016 by The American Society of Hematology.
Figures
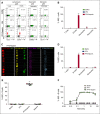

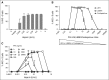
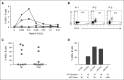
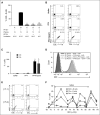

Comment in
-
B cells, PF4/heparin complexes, and complement.Blood. 2016 Oct 6;128(14):1781-1782. doi: 10.1182/blood-2016-08-729517. Blood. 2016. PMID: 28832327 No abstract available.
Similar articles
-
Simultaneous binding of heparin and platelet factor-4 to platelets: further insights into the mechanism of heparin-induced thrombocytopenia.Am J Hematol. 1998 May;58(1):24-30. doi: 10.1002/(sici)1096-8652(199805)58:1<24::aid-ajh5>3.0.co;2-2. Am J Hematol. 1998. PMID: 9590145
-
Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications.Blood. 2006 Mar 15;107(6):2346-53. doi: 10.1182/blood-2005-08-3122. Epub 2005 Nov 22. Blood. 2006. PMID: 16304054 Free PMC article.
-
Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia.Blood. 2011 Jan 27;117(4):1370-8. doi: 10.1182/blood-2010-08-301424. Epub 2010 Oct 19. Blood. 2011. PMID: 20959601
-
Heparin-induced thrombocytopenia: an autoimmune disorder regulated through dynamic autoantigen assembly/disassembly.J Clin Apher. 2007 Feb;22(1):31-6. doi: 10.1002/jca.20109. J Clin Apher. 2007. PMID: 17285619 Review.
-
Antigens involved in heparin-induced thrombocytopenia.Semin Hematol. 1999 Jan;36(1 Suppl 1):7-11. Semin Hematol. 1999. PMID: 9930557 Review.
Cited by
-
Novel Knowledge about Molecular Mechanisms of Heparin-Induced Thrombocytopenia Type II and Treatment Targets.Int J Mol Sci. 2023 May 4;24(9):8217. doi: 10.3390/ijms24098217. Int J Mol Sci. 2023. PMID: 37175923 Free PMC article. Review.
-
Endothelial antigen assembly leads to thrombotic complications in heparin-induced thrombocytopenia.J Clin Invest. 2017 Mar 1;127(3):1090-1098. doi: 10.1172/JCI90958. Epub 2017 Feb 20. J Clin Invest. 2017. PMID: 28218620 Free PMC article.
-
An Update on COVID-19 Vaccine Induced Thrombotic Thrombocytopenia Syndrome and Some Management Recommendations.Molecules. 2021 Aug 18;26(16):5004. doi: 10.3390/molecules26165004. Molecules. 2021. PMID: 34443589 Free PMC article. Review.
-
Fc-modified HIT-like monoclonal antibody as a novel treatment for sepsis.Blood. 2020 Mar 5;135(10):743-754. doi: 10.1182/blood.2019002329. Blood. 2020. PMID: 31722003 Free PMC article.
-
The Crossroads of the Coagulation System and the Immune System: Interactions and Connections.Int J Mol Sci. 2023 Aug 8;24(16):12563. doi: 10.3390/ijms241612563. Int J Mol Sci. 2023. PMID: 37628744 Free PMC article. Review.
References
-
- Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol. 2013;78(2):181–193. - PubMed
-
- Suvarna S, Qi R, Hollingsworth JW, Arepally GM. Platelet factor 4-heparin complexes trigger immune responses independently of the MyD88 pathway. Br J Haematol. 2008;142(4):671–673. - PubMed
-
- Prechel MM, Walenga JM. Complexes of platelet factor 4 and heparin activate Toll-like receptor 4. J Thromb Haemost. 2015;13(4):665–670. - PubMed
-
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

