Agonists and Antagonists of TGF-β Family Ligands
- PMID: 27413100
- PMCID: PMC4968162
- DOI: 10.1101/cshperspect.a021923
Agonists and Antagonists of TGF-β Family Ligands
Abstract
The discovery of the transforming growth factor β (TGF-β) family ligands and the realization that their bioactivities need to be tightly controlled temporally and spatially led to intensive research that has identified a multitude of extracellular modulators of TGF-β family ligands, uncovered their functions in developmental and pathophysiological processes, defined the mechanisms of their activities, and explored potential modulator-based therapeutic applications in treating human diseases. These studies revealed a diverse repertoire of extracellular and membrane-associated molecules that are capable of modulating TGF-β family signals via control of ligand availability, processing, ligand-receptor interaction, and receptor activation. These molecules include not only soluble ligand-binding proteins that were conventionally considered as agonists and antagonists of TGF-β family of growth factors, but also extracellular matrix (ECM) proteins and proteoglycans that can serve as "sink" and control storage and release of both the TGF-β family ligands and their regulators. This extensive network of soluble and ECM modulators helps to ensure dynamic and cell-specific control of TGF-β family signals. This article reviews our knowledge of extracellular modulation of TGF-β growth factors by diverse proteins and their molecular mechanisms to regulate TGF-β family signaling.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
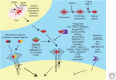
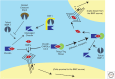

References
-
- Acharya C, Yik JHN, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. 2014. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol 37: 102–111. - PubMed
-
- Agathon A, Thisse B, Thisse C. 2001. Morpholino knock-down of antivin1 and antivin 2 upregulates nodal signaling. Genesis 30: 178–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
