G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons
- PMID: 27413152
- PMCID: PMC4945663
- DOI: 10.1523/JNEUROSCI.3642-15.2016
G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons
Erratum in
-
Erratum: Volpicelli-Daley et al., "G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons".J Neurosci. 2022 Jan 26;42(4):718. doi: 10.1523/JNEUROSCI.2468-21.2021. Epub 2022 Jan 12. J Neurosci. 2022. PMID: 35022221 Free PMC article. No abstract available.
Abstract
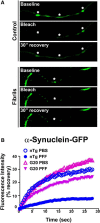
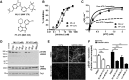
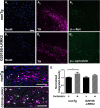
Pathologic inclusions define α-synucleinopathies that include Parkinson's disease (PD). The most common genetic cause of PD is the G2019S LRRK2 mutation that upregulates LRRK2 kinase activity. However, the interaction between α-synuclein, LRRK2, and the formation of α-synuclein inclusions remains unclear. Here, we show that G2019S-LRRK2 expression, in both cultured neurons and dopaminergic neurons in the rat substantia nigra pars compact, increases the recruitment of endogenous α-synuclein into inclusions in response to α-synuclein fibril exposure. This results from the expression of mutant G2019S-LRRK2, as overexpression of WT-LRRK2 not only does not increase formation of inclusions but reduces their abundance. In addition, treatment of primary mouse neurons with LRRK2 kinase inhibitors, PF-06447475 and MLi-2, blocks G2019S-LRRK2 effects, suggesting that the G2019S-LRRK2 potentiation of inclusion formation depends on its kinase activity. Overexpression of G2019S-LRRK2 slightly increases, whereas WT-LRRK2 decreases, total levels of α-synuclein. Knockdown of total α-synuclein with potent antisense oligonucleotides substantially reduces inclusion formation in G2019S-LRRK2-expressing neurons, suggesting that LRRK2 influences α-synuclein inclusion formation by altering α-synuclein levels. These findings support the hypothesis that G2019S-LRRK2 may increase the progression of pathological α-synuclein inclusions after the initial formation of α-synuclein pathology by increasing a pool of α-synuclein that is more susceptible to forming inclusions.
Significance statement: α-Synuclein inclusions are found in the brains of patients with many different neurodegenerative diseases. Point mutation, duplication, or triplication of the α-synuclein gene can all cause Parkinson's disease (PD). The G2019S mutation in LRRK2 is the most common known genetic cause of PD. The interaction between G2019S-LRRK2 and α-synuclein may uncover new mechanisms and targets for neuroprotection. Here, we show that expression of G2019S-LRRK2 increases α-synuclein mobility and enhances aggregation of α-synuclein in primary cultured neurons and in dopaminergic neurons of the substantia nigra pars compacta, a susceptible brain region in PD. Potent LRRK2 kinase inhibitors, which are being developed for clinical use, block the increased α-synuclein aggregation in G2019S-LRRK2-expressing neurons. These results demonstrate that α-synuclein inclusion formation in neurons can be blocked and that novel therapeutic compounds targeting this process by inhibiting LRRK2 kinase activity may slow progression of PD-associated pathology.
Keywords: LRRK2; Lewy body; Lewy neurite; Parkinson's; synuclein.
Copyright © 2016 the authors 0270-6474/16/367416-13$15.00/0.
Figures





Similar articles
-
LRRK2 modifies α-syn pathology and spread in mouse models and human neurons.Acta Neuropathol. 2019 Jun;137(6):961-980. doi: 10.1007/s00401-019-01995-0. Epub 2019 Mar 29. Acta Neuropathol. 2019. PMID: 30927072 Free PMC article.
-
LRRK2 activity does not dramatically alter α-synuclein pathology in primary neurons.Acta Neuropathol Commun. 2018 May 31;6(1):45. doi: 10.1186/s40478-018-0550-0. Acta Neuropathol Commun. 2018. PMID: 29855356 Free PMC article.
-
Inhibition of LRRK2 kinase activity promotes anterograde axonal transport and presynaptic targeting of α-synuclein.Acta Neuropathol Commun. 2021 Nov 8;9(1):180. doi: 10.1186/s40478-021-01283-7. Acta Neuropathol Commun. 2021. PMID: 34749824 Free PMC article.
-
Interaction of LRRK2 and α-Synuclein in Parkinson's Disease.Adv Neurobiol. 2017;14:209-226. doi: 10.1007/978-3-319-49969-7_11. Adv Neurobiol. 2017. PMID: 28353286 Review.
-
α-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of Parkinson disease.Neurotoxicology. 2011 Oct;32(5):622-9. doi: 10.1016/j.neuro.2011.01.003. Epub 2011 Jan 14. Neurotoxicology. 2011. PMID: 21238487 Free PMC article. Review.
Cited by
-
Role of rodent models in advancing precision medicine for Parkinson's disease.Handb Clin Neurol. 2023;193:3-16. doi: 10.1016/B978-0-323-85555-6.00002-3. Handb Clin Neurol. 2023. PMID: 36803818 Free PMC article. Review.
-
The unlikely partnership between LRRK2 and α-synuclein in Parkinson's disease.Eur J Neurosci. 2019 Feb;49(3):339-363. doi: 10.1111/ejn.14182. Epub 2018 Oct 24. Eur J Neurosci. 2019. PMID: 30269383 Free PMC article. Review.
-
Prevention of progression in Parkinson's disease.Biometals. 2018 Oct;31(5):737-747. doi: 10.1007/s10534-018-0131-5. Epub 2018 Jul 20. Biometals. 2018. PMID: 30030679 Free PMC article. Review.
-
α-Synuclein-containing erythrocytic extracellular vesicles: essential contributors to hyperactivation of monocytes in Parkinson's disease.J Neuroinflammation. 2022 Feb 22;19(1):53. doi: 10.1186/s12974-022-02413-1. J Neuroinflammation. 2022. PMID: 35193594 Free PMC article.
-
α-Synuclein pathology in Parkinson's disease and related α-synucleinopathies.Neurosci Lett. 2019 Sep 14;709:134316. doi: 10.1016/j.neulet.2019.134316. Epub 2019 Jun 3. Neurosci Lett. 2019. PMID: 31170426 Free PMC article. Review.
References
-
- Beccano-Kelly DA, Volta M, Munsie LN, Paschall SA, Tatarnikov I, Co K, Chou P, Cao LP, Bergeron S, Mitchell E, Han H, Melrose HL, Tapia L, Raymond LA, Farrer MJ, Milnerwood AJ. LRRK2 overexpression alters glutamatergic presynaptic plasticity, striatal dopamine tone, postsynaptic signal transduction, motor activity and memory. Hum Mol Genet. 2015;24:1336–1349. doi: 10.1093/hmg/ddu543. - DOI - PubMed
-
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. - DOI - PubMed
-
- Boassa D, Berlanga ML, Yang MA, Terada M, Hu J, Bushong EA, Hwang M, Masliah E, George JM, Ellisman MH. Mapping the subcellular distribution of alpha-synuclein in neurons using genetically encoded probes for correlated light and electron microscopy: implications for Parkinson's disease pathogenesis. J Neurosci. 2013;33:2605–2615. doi: 10.1523/JNEUROSCI.2898-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
