A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism
- PMID: 27413154
- PMCID: PMC4945664
- DOI: 10.1523/JNEUROSCI.0636-16.2016
A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism
Abstract
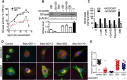
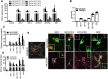
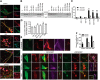
Among the known genetic risk factors for Parkinson disease, mutations in GBA1, the gene responsible for the lysosomal disorder Gaucher disease, are the most common. This genetic link has directed attention to the role of the lysosome in the pathogenesis of parkinsonism. To study how glucocerebrosidase impacts parkinsonism and to evaluate new therapeutics, we generated induced human pluripotent stem cells from four patients with Type 1 (non-neuronopathic) Gaucher disease, two with and two without parkinsonism, and one patient with Type 2 (acute neuronopathic) Gaucher disease, and differentiated them into macrophages and dopaminergic neurons. These cells exhibited decreased glucocerebrosidase activity and stored the glycolipid substrates glucosylceramide and glucosylsphingosine, demonstrating their similarity to patients with Gaucher disease. Dopaminergic neurons from patients with Type 2 and Type 1 Gaucher disease with parkinsonism had reduced dopamine storage and dopamine transporter reuptake. Levels of α-synuclein, a protein present as aggregates in Parkinson disease and related synucleinopathies, were selectively elevated in neurons from the patients with parkinsonism or Type 2 Gaucher disease. The cells were then treated with NCGC607, a small-molecule noninhibitory chaperone of glucocerebrosidase identified by high-throughput screening and medicinal chemistry structure optimization. This compound successfully chaperoned the mutant enzyme, restored glucocerebrosidase activity and protein levels, and reduced glycolipid storage in both iPSC-derived macrophages and dopaminergic neurons, indicating its potential for treating neuronopathic Gaucher disease. In addition, NCGC607 reduced α-synuclein levels in dopaminergic neurons from the patients with parkinsonism, suggesting that noninhibitory small-molecule chaperones of glucocerebrosidase may prove useful for the treatment of Parkinson disease.
Significance statement: Because GBA1 mutations are the most common genetic risk factor for Parkinson disease, dopaminergic neurons were generated from iPSC lines derived from patients with Gaucher disease with and without parkinsonism. These cells exhibit deficient enzymatic activity, reduced lysosomal glucocerebrosidase levels, and storage of glucosylceramide and glucosylsphingosine. Lines generated from the patients with parkinsonism demonstrated elevated levels of α-synuclein. To reverse the observed phenotype, the neurons were treated with a novel noninhibitory glucocerebrosidase chaperone, which successfully restored glucocerebrosidase activity and protein levels and reduced glycolipid storage. In addition, the small-molecule chaperone reduced α-synuclein levels in dopaminergic neurons, indicating that chaperoning glucocerebrosidase to the lysosome may provide a novel therapeutic strategy for both Parkinson disease and neuronopathic forms of Gaucher disease.
Keywords: dopaminergic neurons; glucocerebrosidase; induced pluripotent stem cells; parkinsonism; pharmacological chaperone; α-synuclein.
Copyright © 2016 the authors 0270-6474/16/367442-12$15.00/0.
Figures




References
-
- Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, Marugan J, Patnaik S, Dutra A, Southall N, Zheng W, Tayebi N, Sidransky E. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6:240ra273. doi: 10.1126/scitranslmed.3008659. - DOI - PMC - PubMed
-
- Fernandes HJ, Hartfield EM, Christian HC, Emmanoulidou E, Zheng Y, Booth H, Bogetofte H, Lang C, Ryan BJ, Sardi SP, Badger J, Vowles J, Evetts S, Tofaris GK, Vekrellis K, Talbot K, Hu MT, James W, Cowley SA, Wade-Martins R. ER stress and autophagic perturbations lead to elevated extracellular alpha-synuclein in GBA-N370S Parkinson's iPSC-derived dopamine neurons. Stem Cell Rep. 2016;6:342–356. doi: 10.1016/j.stemcr.2016.01.013. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
