Comparison of Glibenclamide and Insulin on Neonatal Outcomes in Pregnant Women with Gestational Diabetes
- PMID: 27413519
- PMCID: PMC4926540
- DOI: 10.4103/2008-7802.184502
Comparison of Glibenclamide and Insulin on Neonatal Outcomes in Pregnant Women with Gestational Diabetes
Abstract
Background: Untreated or poorly controlled gestational diabetes can cause serious complications for mother and newborn. Glibenclamide is rarely used in treating mothers with this disease. This study aimed at comparing the effect of glibenclamide and insulin on neonatal outcomes in women with gestational diabetes mellitus.
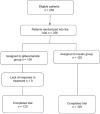
Methods: In this randomized controlled clinical trial, 249 pregnant women aged 18-45 years within the 11(th)-33(rd) weeks of gestation with gestational diabetes, single fetus pregnancy, and in need of hyperglycemia treatment were entered and grouped randomly as either glibenclamide or insulin. In the insulin group (n = 129), insulin was administered with an initial dose of 0.2 IU/kg subcutaneously twice per day, whereas in the glibenclamide group (n = 120), 1.25 mg oral glibenclamide was administered once daily and increased if needed.
Results: The results showed no significant difference in means age, gestational age, and body mass index between women in the two groups. In addition, there were no significant differences in the frequency of neonatal hypoglycemia, anomaly, hyperbilirubinemia, admission in Neonatal Intensive Care Unit (NICU), and neonatal respiratory distress between two groups. Macrosomia was lower in the glibenclamide group than the insulin group (3.3% vs. 13.2%, respectively, P = 0.005). Regression logistics model results showed that the type of treatment (odds ratio [OR]: 4.62; confidence interval [CI]: 1.45-14.02; P = 0.01) and gestational age at delivery (OR: 1.41; CI: 1.04-1.74; P = 0.01) were as predictor factors of macrosomia.
Conclusions: The results of this study revealed that glibenclamide is able to reduce the risk of fetal macrosomia without increasing neonatal anomalies, jaundice, hypocalcemia, infant respiratory distress, and NICU admission.
Keywords: Gestational diabetes; glibenclamide; insulin.
Figures
References
-
- McCance DR. Diabetes in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29:685–99. - PubMed
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. - PubMed
-
- American Diabetes Association. Gestational diabetes mellitus (Position Statement) Diabetes Care. 2004;27(Suppl 1):S88–90. - PubMed
-
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous