Conserved expression of vertebrate microvillar gene homologs in choanocytes of freshwater sponges
- PMID: 27413529
- PMCID: PMC4942974
- DOI: 10.1186/s13227-016-0050-x
Conserved expression of vertebrate microvillar gene homologs in choanocytes of freshwater sponges
Abstract
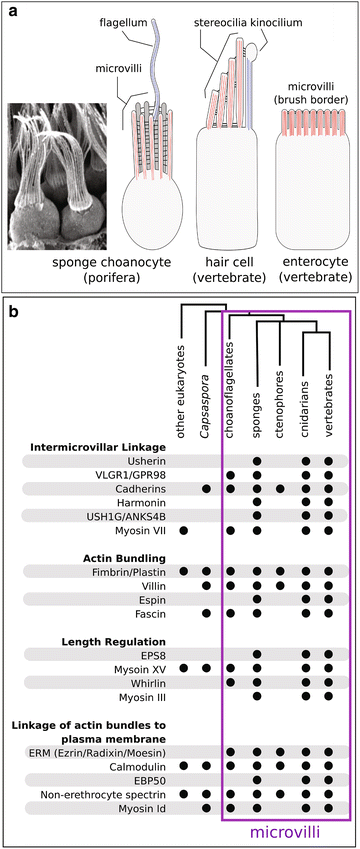
Background: The microvillus is a versatile organelle that serves important functions in disparate animal cell types. However, from a molecular perspective, the microvillus has been well studied in only a few, predominantly vertebrate, contexts. Little is known about how differences in microvillar structure contribute to differences in function, and how these differences evolved. We sequenced the transcriptome of the freshwater sponge, Ephydatia muelleri, and examined the expression of vertebrate microvillar gene homologs in choanocytes-the only microvilli-bearing cell type present in sponges. Sponges offer a distant phylogenetic comparison with vertebrates, and choanocytes are central to discussions about early animal evolution due to their similarity with choanoflagellates, the single-celled sister lineage of modern animals.
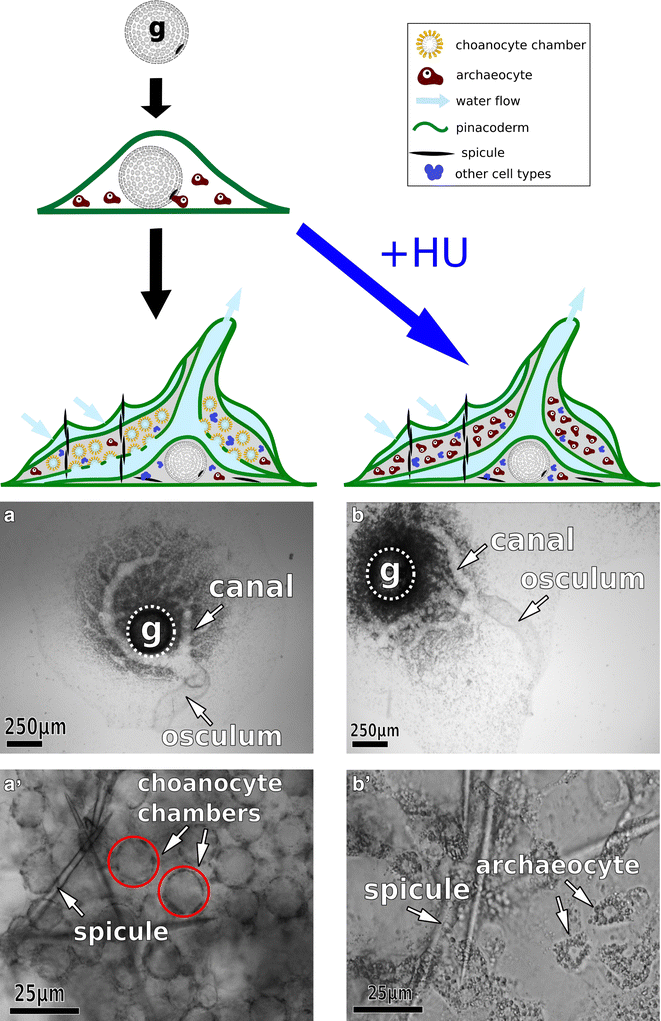
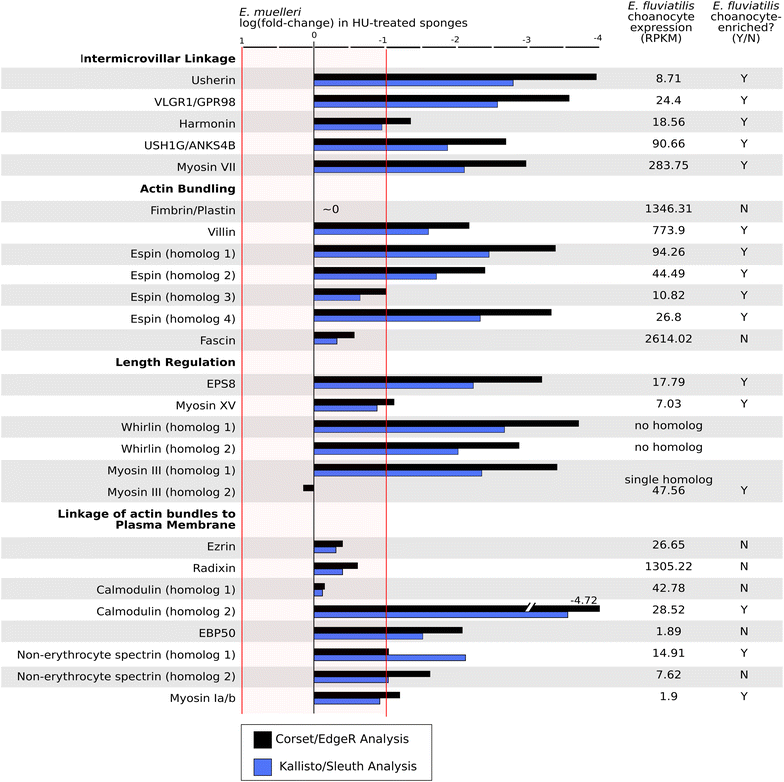
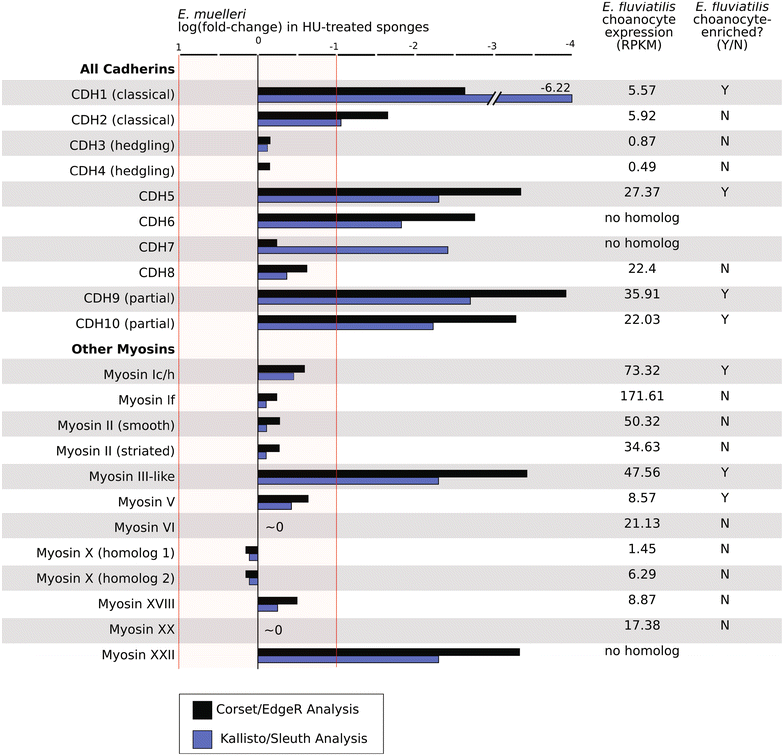
Results: We found that, from a genomic perspective, sponges have conserved homologs of most vertebrate microvillar genes, most of which are expressed in choanocytes, and many of which exhibit choanocyte-specific or choanocyte-enriched expression. Possible exceptions include the cadherins that form intermicrovillar links in the enterocyte brush border and hair cell stereocilia of vertebrates and cnidarians. No obvious orthologs of these proteins were detected in sponges, but at least four candidate cadherins were identified as choanocyte-enriched and might serve this function. In contrast to the evidence for conserved microvillar structure in sponges and vertebrates, we found that choanoflagellates and ctenophores lack homologs of many fundamental microvillar genes, suggesting that microvillar structure may diverge significantly in these lineages, warranting further study.
Conclusions: The available evidence suggests that microvilli evolved early in the prehistory of modern animals and have been repurposed to serve myriad functions in different cellular contexts. Detailed understanding of the sequence by which different microvilli-bearing cell/tissue types diversified will require further study of microvillar composition and development in disparate cell types and lineages. Of particular interest are the microvilli of choanoflagellates, ctenophores, and sponges, which collectively bracket the earliest events in animal evolution.
Keywords: Choanocyte; Ephydatia; Microvilli; Porifera; Sponge; Stereocilia; Transcriptome.
Figures

References
-
- Maldonado M. Choanoflagellates, choanocytes, and animal multicellularity. Invertebr Biol. 2004;123(1):1–22. doi: 10.1111/j.1744-7410.2004.tb00138.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

