Data Quality Monitoring in Clinical Trials: Has It Been Worth It? An Evaluation and Prediction of the Future by All Stakeholders
- PMID: 27413584
- PMCID: PMC4896826
Data Quality Monitoring in Clinical Trials: Has It Been Worth It? An Evaluation and Prediction of the Future by All Stakeholders
Abstract
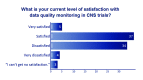
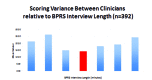
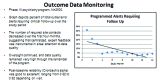
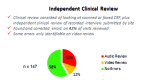
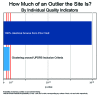
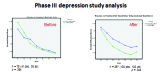
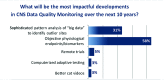
This paper summarizes the results of the CNS Summit Data Quality Monitoring Workgroup analysis of current data quality monitoring techniques used in central nervous system (CNS) clinical trials. Based on audience polls conducted at the CNS Summit 2014, the panel determined that current techniques used to monitor data and quality in clinical trials are broad, uncontrolled, and lack independent verification. The majority of those polled endorse the value of monitoring data. Case examples of current data quality methodology are presented and discussed. Perspectives of pharmaceutical companies and trial sites regarding data quality monitoring are presented. Potential future developments in CNS data quality monitoring are described. Increased utilization of biomarkers as objective outcomes and for patient selection is considered to be the most impactful development in data quality monitoring over the next 10 years. Additional future outcome measures and patient selection approaches are discussed.
Keywords: CNS; Data quality; clinical trial methodology; clinical trials; data monitoring; drug development; trial design.
Figures









LinkOut - more resources
Full Text Sources
