Virus wars: using one virus to block the spread of another
- PMID: 27413636
- PMCID: PMC4933091
- DOI: 10.7717/peerj.2166
Virus wars: using one virus to block the spread of another
Abstract
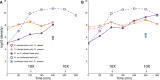
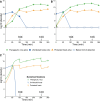
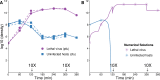
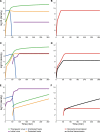
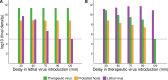
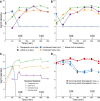
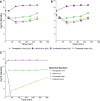
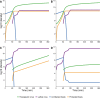
The failure of traditional interventions to block and cure HIV infections has led to novel proposals that involve treating infections with therapeutic viruses-infectious viruses that specifically inhibit HIV propagation in the host. Early efforts in evaluating these proposals have been limited chiefly to mathematical models of dynamics, for lack of suitable empirical systems. Here we propose, develop and analyze an empirical system of a therapeutic virus that protects a host cell population against a lethal virus. The empirical system uses E. coli bacteria as the host cell population, an RNA phage as the lethal virus and a filamentous phage as the therapeutic virus. Basic dynamic properties are established for each virus alone and then together. Observed dynamics broadly agree with those predicted by a computer simulation model, although some differences are noted. Two cases of dynamics are contrasted, differing in whether the therapeutic virus is introduced before the lethal virus or after the lethal virus. The therapeutic virus increases in both cases but by different mechanisms. With the therapeutic virus introduced first, it spreads infectiously without any appreciable change in host dynamics. With the therapeutic virus introduced second, host abundance is depressed at the time therapy is applied; following an initial period of therapeutic virus spread by infection, the subsequent rise of protection is through reproduction by hosts already protected. This latter outcome is due to inheritance of the therapeutic virus state when the protected cell divides. Overall, the work establishes the feasibility and robustness to details of a viral interference using a therapeutic virus.
Keywords: Bacteriophage; Gene therapy; Infectious vaccine; Intervention; Mathematical model; Population dynamics; Vaccine alternative.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
References
-
- Adams MH. Bacteriophages. Interscience Publishers; New York: 1959.
-
- Bohannan B, Lenski R. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecology Letters. 2000;3(4):362–377. doi: 10.1046/j.1461-0248.2000.00161.x. - DOI
-
- Brown NJ, Hirsch ML. Adeno-associated virus (AAV) gene delivery in stem cell therapy. Discovery Medicine. 2015;20(111):333–342. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

