Further results of the FRAGMATIC trial of thromboprophylaxis in lung cancer
- PMID: 27413715
- PMCID: PMC4931121
- DOI: 10.21037/tlcr.2016.05.07
Further results of the FRAGMATIC trial of thromboprophylaxis in lung cancer
Conflict of interest statement
Figures
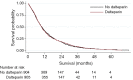
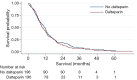
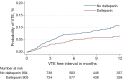
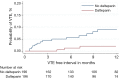
Comment on
- Transl Lung Cancer Res. 5:280.
References
-
- Noble S, Robbins A, Alikhan R, et al. Prediction of venous thromboembolism in lung cancer patients receiving chemotherapy. Journal of the International Society of Thrombosis and Haemostasis 2015;13:143.
LinkOut - more resources
Full Text Sources
Other Literature Sources
