Preexisting Antibodies to an F(ab')2 Antibody Therapeutic and Novel Method for Immunogenicity Assessment
- PMID: 27413757
- PMCID: PMC4927981
- DOI: 10.1155/2016/2921758
Preexisting Antibodies to an F(ab')2 Antibody Therapeutic and Novel Method for Immunogenicity Assessment
Abstract
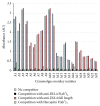
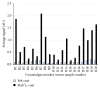
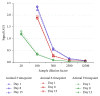
Anti-therapeutic antibodies (ATAs) may impact drug exposure and activity and induce immune complex mediated toxicity; therefore the accurate measurement of ATA is important for the analysis of drug safety and efficacy. Preexisting ATAs to the hinge region of anti-Delta like ligand 4 (anti-DLL4) F(ab')2, a potential antitumor therapeutic, were detected in cynomolgus monkey serum, which presented a challenge in developing assays for detecting treatment induced ATA. A total ATA assay was developed using a bridging ELISA that detected both anti-CDR and anti-framework ATA including anti-hinge reactivity. A competition assay that could detect 500 ng/mL of anti-CDR ATA in the presence of preexisting ATA was also developed to determine ATA specific to the anti-DLL4 F(ab')2 CDR using anti-DLL4 F(ab')2 and a control F(ab')2. We used these assay methods in a cynomolgus monkey in vivo study to successfully evaluate total and anti-CDR ATA. The preexisting anti-hinge reactivity was also observed to a lesser extent in human serum, and a similar approach could be applied for specific immunogenicity assessment in clinical trials.
Figures


Similar articles
-
A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo.MAbs. 2014;6(5):1265-73. doi: 10.4161/mabs.29825. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517311 Free PMC article.
-
Epitope characterization of pre-existing and developing antibodies to an aglycosylated monoclonal antibody therapeutic of G1m17,1 allotype.J Immunol Methods. 2012 Aug 31;382(1-2):93-100. doi: 10.1016/j.jim.2012.05.009. Epub 2012 May 15. J Immunol Methods. 2012. PMID: 22609464 Clinical Trial.
-
Development and validation of an antigen-binding capture ELISA for native and putrescine-modified anti-tetanus F(ab')2 fragments for the assessment of the cellular uptake and plasma kinetics of the antibodies.J Immunol Methods. 2005 Dec 20;307(1-2):82-95. doi: 10.1016/j.jim.2005.09.015. Epub 2005 Nov 4. J Immunol Methods. 2005. PMID: 16305797
-
Stimulation of complement amplification by F(ab')(2)-containing immune complexes and naturally occurring anti-hinge antibodies, possible role in systemic inflammation.Autoimmun Rev. 2008 Jun;7(6):508-13. doi: 10.1016/j.autrev.2008.04.017. Epub 2008 May 12. Autoimmun Rev. 2008. PMID: 18558371 Review.
-
Emerging Technologies and Generic Assays for the Detection of Anti-Drug Antibodies.J Immunol Res. 2016;2016:6262383. doi: 10.1155/2016/6262383. Epub 2016 Jul 31. J Immunol Res. 2016. PMID: 27556048 Free PMC article. Review.
Cited by
-
Proteolytic single hinge cleavage of pertuzumab impairs its Fc effector function and antitumor activity in vitro and in vivo.Breast Cancer Res. 2018 Jun 1;20(1):43. doi: 10.1186/s13058-018-0972-4. Breast Cancer Res. 2018. PMID: 29859099 Free PMC article.
-
Recommendations for the Assessment and Management of Pre-existing Drug-Reactive Antibodies During Biotherapeutic Development.AAPS J. 2017 Nov;19(6):1576-1586. doi: 10.1208/s12248-017-0153-x. Epub 2017 Nov 6. AAPS J. 2017. PMID: 29110222
-
Proteinase-nicked IgGs: an unanticipated target for tumor immunotherapy.Oncoimmunology. 2018 Jul 23;7(9):e1480300. doi: 10.1080/2162402X.2018.1480300. eCollection 2018. Oncoimmunology. 2018. PMID: 30228951 Free PMC article. Review.
-
Fragment-Based Immune Cell Engager Antibodies in Treatment of Cancer, Infectious and Autoimmune Diseases: Lessons and Insights from Clinical and Translational Studies.Antibodies (Basel). 2025 Jun 24;14(3):52. doi: 10.3390/antib14030052. Antibodies (Basel). 2025. PMID: 40700292 Free PMC article. Review.
References
-
- U.S. Department of Health and Human Services. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products, 2014.
-
- European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. Doc. Ref. 2007;(EMEA/CHMP/BMWP/14327/2006)
-
- European Medicines Agency. Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins intended for in-vivo clinical use. Doc. Ref. 2012;(EMA/CHMP/BMWP/86289/2010)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

