Anion-π Enzymes
- PMID: 27413782
- PMCID: PMC4919773
- DOI: 10.1021/acscentsci.6b00097
Anion-π Enzymes
Abstract
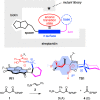
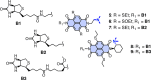
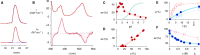
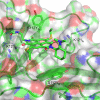
In this report, we introduce artificial enzymes that operate with anion-π interactions, an interaction that is essentially new to nature. The possibility to stabilize anionic intermediates and transition states on an π-acidic surface has been recently demonstrated, using the addition of malonate half thioesters to enolate acceptors as a biologically relevant example. The best chiral anion-π catalysts operate with an addition/decarboxylation ratio of 4:1, but without any stereoselectivity. To catalyze this important but intrinsically disfavored reaction stereoselectively, a series of anion-π catalysts was equipped with biotin and screened against a collection of streptavidin mutants. With the best hit, the S112Y mutant, the reaction occurred with 95% ee and complete suppression of the intrinsically favored side product from decarboxylation. This performance of anion-π enzymes rivals, if not exceeds, that of the best conventional organocatalysts. Inhibition of the S112Y mutant by nitrate but not by bulky anions supports that contributions from anion-π interactions exist and matter, also within proteins. In agreement with docking results, K121 is shown to be essential, presumably to lower the pK a of the tertiary amine catalyst to operate at the optimum pH around 3, that is below the pK a of the substrate. Most importantly, increasing enantioselectivity with different mutants always coincides with increasing rates and conversion, i.e., selective transition-state stabilization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

