Transdifferentiation and Proliferation in Two Distinct Hemocyte Lineages in Drosophila melanogaster Larvae after Wasp Infection
- PMID: 27414410
- PMCID: PMC4945071
- DOI: 10.1371/journal.ppat.1005746
Transdifferentiation and Proliferation in Two Distinct Hemocyte Lineages in Drosophila melanogaster Larvae after Wasp Infection
Abstract
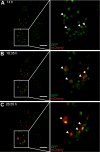
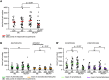
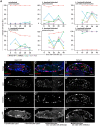
Cellular immune responses require the generation and recruitment of diverse blood cell types that recognize and kill pathogens. In Drosophila melanogaster larvae, immune-inducible lamellocytes participate in recognizing and killing parasitoid wasp eggs. However, the sequence of events required for lamellocyte generation remains controversial. To study the cellular immune system, we developed a flow cytometry approach using in vivo reporters for lamellocytes as well as for plasmatocytes, the main hemocyte type in healthy larvae. We found that two different blood cell lineages, the plasmatocyte and lamellocyte lineages, contribute to the generation of lamellocytes in a demand-adapted hematopoietic process. Plasmatocytes transdifferentiate into lamellocyte-like cells in situ directly on the wasp egg. In parallel, a novel population of infection-induced cells, which we named lamelloblasts, appears in the circulation. Lamelloblasts proliferate vigorously and develop into the major class of circulating lamellocytes. Our data indicate that lamellocyte differentiation upon wasp parasitism is a plastic and dynamic process. Flow cytometry with in vivo hemocyte reporters can be used to study this phenomenon in detail.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

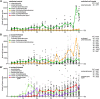
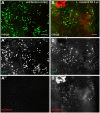

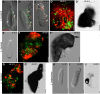

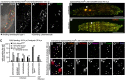

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

