ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells
- PMID: 27415012
- PMCID: PMC4985349
- DOI: 10.1038/bjc.2016.203
ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells
Abstract
Background: Clinical response to chemotherapy for ovarian cancer is frequently compromised by the development of drug-resistant disease. The underlying molecular mechanisms and implications for prescription of routinely prescribed chemotherapy drugs are poorly understood.
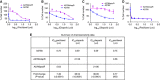
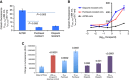
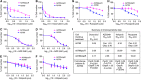
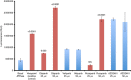
Methods: We created novel A2780-derived ovarian cancer cell lines resistant to paclitaxel and olaparib following continuous incremental drug selection. MTT assays were used to assess chemosensitivity to paclitaxel and olaparib in drug-sensitive and drug-resistant cells±the ABCB1 inhibitors verapamil and elacridar and cross-resistance to cisplatin, carboplatin, doxorubicin, rucaparib, veliparib and AZD2461. ABCB1 expression was assessed by qRT-PCR, copy number, western blotting and immunohistochemical analysis and ABCB1 activity assessed by the Vybrant and P-glycoprotein-Glo assays.
Results: Paclitaxel-resistant cells were cross-resistant to olaparib, doxorubicin and rucaparib but not to veliparib or AZD2461. Resistance correlated with increased ABCB1 expression and was reversible following treatment with the ABCB1 inhibitors verapamil and elacridar. Active efflux of paclitaxel, olaparib, doxorubicin and rucaparib was confirmed in drug-resistant cells and in ABCB1-expressing bacterial membranes.
Conclusions: We describe a common ABCB1-mediated mechanism of paclitaxel and olaparib resistance in ovarian cancer cells. Optimal choice of PARP inhibitor may therefore limit the progression of drug-resistant disease, while routine prescription of first-line paclitaxel may significantly limit subsequent chemotherapy options in ovarian cancer patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ahmed AA, Mills AD, Ibrahim AE, Temple J, Blenkiron C, Vias M, Massie CE, Iyer NG, McGeoch A, Crawford R, Nicke B, Downward J, Swanton C, Bell SD, Earl HM, Laskey RA, Caldas C, Brenton JD (2007) The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 12(6): 514–527. - PMC - PubMed
-
- Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH (2000) Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res 60(20): 5761–5766. - PubMed
-
- Arao S, Suwa H, Mandai M, Tashiro H, Miyazaki K, Okamura H, Nomura H, Hiai H, Fukumoto M (1994) Expression of multidrug resistance gene and localization of P-glycoprotein in human primary ovarian cancer. Cancer Res 54(5): 1355–1359. - PubMed
-
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376(9737): 245–251. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

