C4-Dicarboxylate Utilization in Aerobic and Anaerobic Growth
- PMID: 27415771
- PMCID: PMC11575717
- DOI: 10.1128/ecosalplus.ESP-0021-2015
C4-Dicarboxylate Utilization in Aerobic and Anaerobic Growth
Abstract
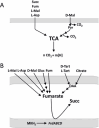
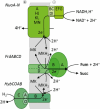
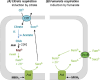
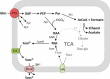
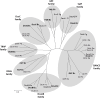
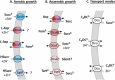
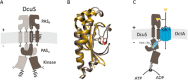
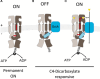
C4-dicarboxylates and the C4-dicarboxylic amino acid l-aspartate support aerobic and anaerobic growth of Escherichia coli and related bacteria. In aerobic growth, succinate, fumarate, D- and L-malate, L-aspartate, and L-tartrate are metabolized by the citric acid cycle and associated reactions. Because of the interruption of the citric acid cycle under anaerobic conditions, anaerobic metabolism of C4-dicarboxylates depends on fumarate reduction to succinate (fumarate respiration). In some related bacteria (e.g., Klebsiella), utilization of C4-dicarboxylates, such as tartrate, is independent of fumarate respiration and uses a Na+-dependent membrane-bound oxaloacetate decarboxylase. Uptake of the C4-dicarboxylates into the bacteria (and anaerobic export of succinate) is achieved under aerobic and anaerobic conditions by different sets of secondary transporters. Expression of the genes for C4-dicarboxylate metabolism is induced in the presence of external C4-dicarboxylates by the membrane-bound DcuS-DcuR two-component system. Noncommon C4-dicarboxylates like l-tartrate or D-malate are perceived by cytoplasmic one-component sensors/transcriptional regulators. This article describes the pathways of aerobic and anaerobic C4-dicarboxylate metabolism and their regulation. The citric acid cycle, fumarate respiration, and fumarate reductase are covered in other articles and discussed here only in the context of C4-dicarboxylate metabolism. Recent aspects of C4-dicarboxylate metabolism like transport, sensing, and regulation will be treated in more detail. This article is an updated version of an article published in 2004 in EcoSal Plus. The update includes new literature, but, in particular, the sections on the metabolism of noncommon C4-dicarboxylates and their regulation, on the DcuS-DcuR regulatory system, and on succinate production by engineered E. coli are largely revised or new.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

