Linear transformation of the encoding mechanism for light intensity underlies the paradoxical enhancement of cortical visual responses by sevoflurane
- PMID: 27416731
- PMCID: PMC5199737
- DOI: 10.1113/JP272215
Linear transformation of the encoding mechanism for light intensity underlies the paradoxical enhancement of cortical visual responses by sevoflurane
Abstract
Key points: The mechanisms of action of anaesthetics on the living brain are still poorly understood. In this respect, the analysis of the differential effects of anaesthetics on spontaneous and sensory-evoked cortical activity might provide important and novel cues. Here we show that the anaesthetic sevoflurane strongly silences the brain but potentiates in a dose- and frequency-dependent manner the cortical visual response. Such enhancement arises from a linear scaling by sevoflurane of the power-law relation between light intensity and the cortical response. The fingerprint of sevoflurane action suggests that circuit silencing can boost linearly synaptic responsiveness presumably by scaling the number of responding units and/or their correlation following a sensory stimulation.
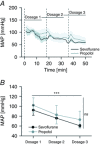
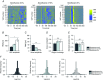
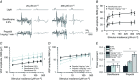
Abstract: General anaesthetics, which are expected to silence brain activity, often spare sensory responses. To evaluate differential effects of anaesthetics on spontaneous and sensory-evoked cortical activity, we characterized their modulation by sevoflurane and propofol. Power spectra and the bust-suppression ratio from EEG data were used to evaluate anaesthesia depth. ON and OFF cortical responses were elicited by light pulses of variable intensity, duration and frequency, during light and deep states of anaesthesia. Both anaesthetics reduced spontaneous cortical activity but sevoflurane greatly enhanced while propofol diminished the ON visual response. Interestingly, the large potentiation of the ON visual response by sevoflurane was found to represent a linear scaling of the encoding mechanism for light intensity. To the contrary, the OFF cortical visual response was depressed by both anaesthetics. The selective depression of the OFF component by sevoflurane could be converted into a robust potentiation by the pharmacological blockade of the ON pathway, suggesting that the temporal order of ON and OFF responses leads to a depression of the latter. This hypothesis agrees with the finding that the enhancement of the ON response was converted into a depression by increasing the frequency of light-pulse stimulation from 0.1 to 1 Hz. Overall, our results support the view that inactivity-dependent modulation of cortical circuits produces an increase in their responsiveness. Among the implications of our findings, the silencing of cortical circuits can boost linearly the cortical responsiveness but with negative impact on their frequency transfer and with a loss of the information content of the sensory signal.
Keywords: anaesthesia; cortical silencing; gain modulation; inactivity-dependent modulation; intensity sensitivity; visual cortex; visual evoked potential.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures





References
-
- Asahi T, Hirota K, Sasaki R, Mitsuaki Y & Roth SH (2006). Intravenous anesthetics are more effective than volatile anesthetics on inhibitory pathways in rat hippocampal CA1. Anesth Analg 102, 772–778. - PubMed
-
- Ayaz A & Chance FS (2009). Gain modulation of neuronal responses by subtractive and divisive mechanisms of inhibition. J Neurophysiol 101, 958–968. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

