Cochrane: the unfinished symphony of research synthesis
- PMID: 27416925
- PMCID: PMC4946116
- DOI: 10.1186/s13643-016-0290-9
Cochrane: the unfinished symphony of research synthesis
Abstract
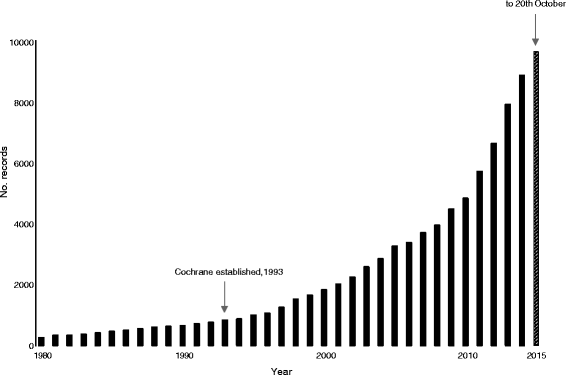
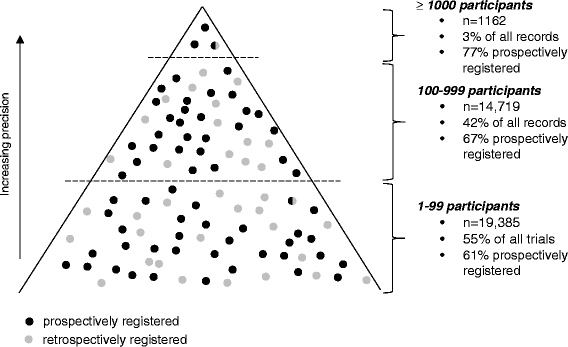
The NHS needs valid information on the safety and effectiveness of healthcare interventions. Cochrane systematic reviews are an important source of this information. Traditionally, Cochrane has attempted to identify and include all relevant trials in systematic reviews on the basis that if all trials are identified and included, there should be no selection bias. However, a predictable consequence of the drive to include all trials is that some studies are included that are not trials (false positives). Including such studies in reviews might increase bias. More effort is needed to authenticate trials to be included in reviews, but this task is bedevilled by the enormous increase in the number of 'trials' conducted each year. We argue that excluding small trials from reviews would release resources for more detailed appraisal of larger trials. Conducting fewer but broader reviews that contain fewer but properly validated trials might better serve patients' interests.
Figures
References
-
- http://www.alltrials.net/. (Accessed 29 Jan 2015)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources