Evaluation of a Micro-Optical Coherence Tomography for the Corneal Endothelium in an Animal Model
- PMID: 27416929
- PMCID: PMC4945948
- DOI: 10.1038/srep29769
Evaluation of a Micro-Optical Coherence Tomography for the Corneal Endothelium in an Animal Model
Abstract
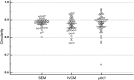
Recent developments in optical coherence tomography (OCT) systems for the cornea have limited resolution or acquisition speed. In this study we aim to evaluate the use of a 'micro-OCT' (μOCT ~1 μm axial resolution) compared to existing imaging modalities using animal models of corneal endothelial disease. We used established cryoinjury and bullous keratopathy models in Sprague Dawley rats comparing ex vivo μOCT imaging in normal and diseased eyes to (1) histology; (2) in vivo confocal microscopy (IVCM); and (3) scanning electron microscopy (SEM). Qualitative and quantitative comparisons amongst imaging modalities were performed using mean endothelial cell circularity [(4π × Area)/Perimeter(2)] with coefficient of variation (COV). We found that μOCT imaging was able to delineate endothelial cells (with nuclei), detect inflammatory cells, and corneal layers with histology-like resolution, comparable to existing imaging modalities. The mean endothelial cell circularity score was 0.88 ± 0.03, 0.87 ± 0.04 and 0.88 ± 0.05 (P = 0.216) for the SEM, IVCM and μOCT respectively, with SEM producing homogenous endothelial cell images (COV = 0.028) compared to the IVCM (0.051) and μOCT (0.062). In summary, our preliminary study suggests that the μOCT may be useful for achieving non-contact, histology-like images of the cornea for endothelial cell evaluation, which requires further development for in vivo imaging.
Figures



References
-
- Foster A. & Resnikoff S. The impact of Vision 2020 on global blindness. Eye (Lond) 19, 1133–1135 (2005). - PubMed
-
- Tan D. T., Dart J. K., Holland E. J. & Kinoshita S. Corneal transplantation. Lancet 379, 1749–1761 (2012). - PubMed
-
- Ang M. et al.. Endothelial cell loss and graft survival after Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology 119, 2239–2244 (2012). - PubMed
-
- Han D. C., Mehta J. S., Por Y. M., Htoon H. M. & Tan D. T. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol 148, 744–751 e741 (2009). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

