IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation
- PMID: 27417023
- PMCID: PMC5149451
- DOI: 10.1016/j.jaci.2016.04.037
IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation
Abstract
Background: Increased IL-17A production has been associated with more severe asthma; however, the mechanisms whereby IL-17A can contribute to IL-13-driven pathology in asthmatic patients remain unclear.
Objective: We sought to gain mechanistic insight into how IL-17A can influence IL-13-driven responses.
Methods: The effect of IL-17A on IL-13-induced airway hyperresponsiveness, gene expression, mucus hypersecretion, and airway inflammation was assessed by using in vivo models of IL-13-induced lung pathology and in vitro culture of murine fibroblast cell lines and primary fibroblasts and human epithelial cell lines or primary human epithelial cells exposed to IL-13, IL-17A, or both.
Results: Compared with mice given intratracheal IL-13 alone, those exposed to IL-13 and IL-17A had augmented airway hyperresponsiveness, mucus production, airway inflammation, and IL-13-induced gene expression. In vitro, IL-17A enhanced IL-13-induced gene expression in asthma-relevant murine and human cells. In contrast to the exacerbating influence of IL-17A on IL-13-induced responses, coexposure to IL-13 inhibited IL-17A-driven antimicrobial gene expression in vivo and in vitro. Mechanistically, in both primary human and murine cells, the IL-17A-driven increase in IL-13-induced gene expression was associated with enhanced IL-13-driven signal transducer and activator of transcription 6 activation.
Conclusions: Our data suggest that IL-17A contributes to asthma pathophysiology by increasing the capacity of IL-13 to activate intracellular signaling pathways, such as signal transducer and activator of transcription 6. These data represent the first mechanistic explanation of how IL-17A can directly contribute to the pathogenesis of IL-13-driven pathology.
Keywords: Asthma; IL-13; IL-17A; cytokines; signal transducer and activator of transcription 6; signal transduction.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

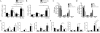
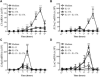

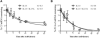

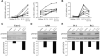
References
-
- Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–75. - PubMed
-
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. - PubMed
-
- Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

