Effects of the Nrf2 Protein Modulator Salvianolic Acid A Alone or Combined with Metformin on Diabetes-associated Macrovascular and Renal Injury
- PMID: 27417135
- PMCID: PMC5064007
- DOI: 10.1074/jbc.M115.712703
Effects of the Nrf2 Protein Modulator Salvianolic Acid A Alone or Combined with Metformin on Diabetes-associated Macrovascular and Renal Injury
Abstract
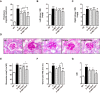
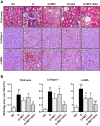
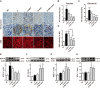
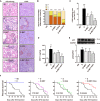
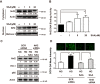
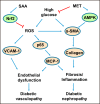
Nuclear factor E2-related factor 2 (Nrf2) is considered a promising target against diabetic complications such as cardiovascular diseases and diabetic nephropathy. Herein, we investigated the effects of a potential Nrf2 modulator, salvianolic acid A (SAA), which is a natural polyphenol, on diabetes-associated macrovascular and renal injuries in streptozotocin-induced diabetic mice. Given that lowering glucose is the first objective of diabetic patients, we also examined the effects of SAA combined with metformin (MET) on both complications. Our results showed that SAA significantly increased the macrovascular relaxation response to acetylcholine and sodium nitroprusside in diabetic mice. Interestingly, treatment with SAA alone only provided minor protection against renal injury, as reflected by minor improvements in impaired renal function and structure, despite significantly reduced oxidative stress observed in the diabetic kidney. We demonstrated that decreased oxidative stress and NF-κB p65 expression were associated with SAA-induced expression of Nrf2-responsive antioxidant enzymes heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase (quinone) 1 (NQO-1), and glutathione peroxidase-1 (GPx-1) in vivo or in vitro, which suggested that SAA was a potential Nrf2 modulator. More significantly, compared with treatment with either SAA or MET alone, we found that their combination provided further protection against the macrovascular and renal injury, which was at least partly due to therapeutic activation of both MET-mediated AMP-activated protein kinase and SAA-mediated Nrf2/antioxidant-response element pathways. These findings suggested that polyphenol Nrf2 modulators, especially combined with drugs activating AMP-activated protein kinase, including hypoglycemic drugs, are worthy of further investigation to combat diabetic complications.
Keywords: Nuclear factor 2 (erythroid-derived 2-like factor) (NFE2L2) (Nrf2); diabetes; diabetic macrovascular injury; diabetic nephropathy; metformin; oxidative stress; salvianolic acid A.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

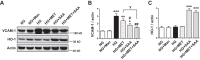

Similar articles
-
Salvia miltiorrhiza Lipophilic Fraction Attenuates Oxidative Stress in Diabetic Nephropathy through Activation of Nuclear Factor Erythroid 2-Related Factor 2.Am J Chin Med. 2017;45(7):1441-1457. doi: 10.1142/S0192415X17500781. Epub 2017 Sep 25. Am J Chin Med. 2017. PMID: 28946766
-
Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3β activity and activating the Nrf2/HO-1 pathway.Phytomedicine. 2022 Jan;95:153856. doi: 10.1016/j.phymed.2021.153856. Epub 2021 Nov 16. Phytomedicine. 2022. PMID: 34856477
-
Coptisine ameliorates renal injury in diabetic rats through the activation of Nrf2 signaling pathway.Naunyn Schmiedebergs Arch Pharmacol. 2020 Jan;393(1):57-65. doi: 10.1007/s00210-019-01710-6. Epub 2019 Aug 16. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31420722
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats.Food Chem Toxicol. 2014 Sep;71:116-21. doi: 10.1016/j.fct.2014.05.023. Epub 2014 Jun 4. Food Chem Toxicol. 2014. PMID: 24907624 Review.
Cited by
-
Baicalein pre-treatment alleviates hepatic ischemia/reperfusion injury in mice by regulating the Nrf2/ARE pathway.Exp Ther Med. 2021 Dec;22(6):1380. doi: 10.3892/etm.2021.10816. Epub 2021 Sep 28. Exp Ther Med. 2021. PMID: 34650628 Free PMC article.
-
Antioxidant effects of astaxanthin and metformin combined therapy in type 2 diabetes mellitus patients: a randomized double-blind controlled clinical trial.Res Pharm Sci. 2022 Jan 15;17(2):219-230. doi: 10.4103/1735-5362.335179. eCollection 2022 Apr. Res Pharm Sci. 2022. PMID: 35280834 Free PMC article.
-
Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing.Front Bioeng Biotechnol. 2021 Jun 24;9:707479. doi: 10.3389/fbioe.2021.707479. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34249895 Free PMC article. Review.
-
Salvianolic Acid A Ameliorates Early-Stage Atherosclerosis Development by Inhibiting NLRP3 Inflammasome Activation in Zucker Diabetic Fatty Rats.Molecules. 2020 Feb 28;25(5):1089. doi: 10.3390/molecules25051089. Molecules. 2020. PMID: 32121151 Free PMC article.
-
Exploration of metformin-based drug combination for mitigating diabetes-associated atherosclerotic diseases.World J Diabetes. 2025 Apr 15;16(4):100533. doi: 10.4239/wjd.v16.i4.100533. World J Diabetes. 2025. PMID: 40236872 Free PMC article.
References
-
- Jaiswal A. K. (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol Med. 36, 1199–1207 - PubMed
-
- Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., and Yamamoto M. (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 29, 493–502 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

