Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease
- PMID: 27417573
- PMCID: PMC4945922
- DOI: 10.1038/srep29783
Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease
Abstract
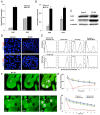
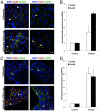
Inflammatory bowel disease (IBD) involves functional impairment of intestinal epithelial cells (IECs), concomitant with the infiltration of the lamina propria by inflammatory cells. We explored the reciprocal paracrine and direct interaction between human IECs and macrophages (MΦ) in a co-culture system that mimics some aspects of IBD. We investigated the expression of intercellular junctional proteins in cultured IECs under inflammatory conditions and in tissues from IBD patients. IECs establish functional gap junctions with IECs and MΦ, respectively. Connexin (Cx26) and Cx43 expression in cultured IECs is augmented under inflammatory conditions; while, Cx43-associated junctional complexes partners, E-cadherin, ZO-1, and β-catenin expression is decreased. The expression of Cx26 and Cx43 in IBD tissues is redistributed to the basal membrane of IEC, which is associated with decrease in junctional complex proteins' expression, collagen type IV expression and infiltration of MΦ. These data support the notion that the combination of paracrine and hetero-cellular communication between IECs and MΦs may regulate epithelial cell function through the establishment of junctional complexes between inflammatory cells and IECs, which ultimately contribute to the dys-regulation of intestinal epithelial barrier.
Figures





References
-
- Fiocchi C. Intestinal inflammation: a complex interplay of immune and interactions. Am. J. Physiol. 273, G769–75 (1997). - PubMed
-
- Henderson P., van Limbergen J. E., Schwarze J. & Wilson D. C. Function of the Intestinal Epithelium and Its Dysregulation in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 17, 382–395 (2011). - PubMed
-
- De Maio A., Vega V. L. & Contreras J. E. Gap junctions, homeostasis, and injury. J. Cell Physiol. 191, 269–82 (2002). - PubMed
-
- Musil L. S., Le A. C., VanSlyke J. K. & Roberts L. M. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J. Biol. Chem. 275, 25207–15 (2000). - PubMed
-
- Segretaina D. & Falk M. M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochimica et Biophysica Acta. 1662, 3–21 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

