The Blood and Marrow Transplant Clinical Trials Network: An Effective Infrastructure for Addressing Important Issues in Hematopoietic Cell Transplantation
- PMID: 27418009
- PMCID: PMC5027144
- DOI: 10.1016/j.bbmt.2016.07.003
The Blood and Marrow Transplant Clinical Trials Network: An Effective Infrastructure for Addressing Important Issues in Hematopoietic Cell Transplantation
Abstract
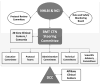
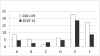
Hematopoietic cell transplantation (HCT) is a rapidly evolving field with active preclinical and clinical development of new strategies for patient assessment, graft selection and manipulation, and pre- and post-transplantation drug and cell therapy. New strategies require evaluation in definitive clinical trials; however, HCT trials face unique challenges, including the relatively small number of transplantations performed at any single center, the diverse indications for HCT requiring dissimilar approaches, the complex nature of the intervention itself, the risk of multiple complications in the immediate post-transplantation period, and the risk of important, though infrequent, late effects. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) was established by the US National Heart Lung and Blood Institute and the National Cancer Institute to meet these challenges. In its 15 years as a network, the BMT CTN has proven to be a successful infrastructure for planning, implementing, and completing such trials and for providing definitive answers to questions leading to improvements in the understanding and practice of HCT. It has opened 37 trials, about one-half phase 2 and one-half phase 3, enrolled more than 8000 patients, and published 57 papers addressing important issues in the treatment of patients with life-threatening malignant and nonmalignant blood disorders. This review describes the network's accomplishments, key components of its success, lessons learned over the past 15 years, and challenges for the future.
Keywords: Blood and Marrow Transplant Clinical Trials Network; Hematopoietic cell transplantation; Infrastructure; Review.
Copyright © 2016 The American Society for Blood and Marrow Transplantation. All rights reserved.
Figures

References
-
- O’Reilly RJ. Clinical Trials of Hematopoietic Cell Transplantations: Current Needs and Future Strategies. Biol Blood Marrow Transplant. 2000;6(2):79–89. - PubMed
-
- Weisdorf D, Carter S, Confer D, et al. Blood and Marrow Transplant Clinical Trials Network (BMT CTN): Addressing unanswered questions. Biol Blood Marrow Transplant. 2007;13(3):257–262. discussion 255–256. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- U10 HL109526/HL/NHLBI NIH HHS/United States
- U10 HL069254/HL/NHLBI NIH HHS/United States
- U10 HL069290/HL/NHLBI NIH HHS/United States
- U10 HL069301/HL/NHLBI NIH HHS/United States
- U10 HL069330/HL/NHLBI NIH HHS/United States
- U10 HL069291/HL/NHLBI NIH HHS/United States
- U10 HL069310/HL/NHLBI NIH HHS/United States
- U01 HL069294/HL/NHLBI NIH HHS/United States
- R01 HL069256/HL/NHLBI NIH HHS/United States
- U10 HL069315/HL/NHLBI NIH HHS/United States
- U10 HL069348/HL/NHLBI NIH HHS/United States
- U10 HL069274/HL/NHLBI NIH HHS/United States
- U10 HL069278/HL/NHLBI NIH HHS/United States
- U10 HL069233/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U10 HL069286/HL/NHLBI NIH HHS/United States
- UG1 HL069278/HL/NHLBI NIH HHS/United States
- U10 HL069249/HL/NHLBI NIH HHS/United States
- U10 HL069334/HL/NHLBI NIH HHS/United States
- U10 HL109137/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 HL109322/HL/NHLBI NIH HHS/United States
- U10 HL108945/HL/NHLBI NIH HHS/United States
- U10 HL108987/HL/NHLBI NIH HHS/United States
- U01 HL069273/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

