Structural investigation of heteroyohimbine alkaloid synthesis reveals active site elements that control stereoselectivity
- PMID: 27418042
- PMCID: PMC4947188
- DOI: 10.1038/ncomms12116
Structural investigation of heteroyohimbine alkaloid synthesis reveals active site elements that control stereoselectivity
Abstract
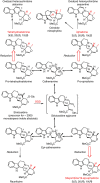
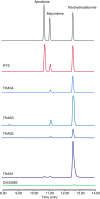
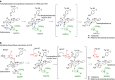
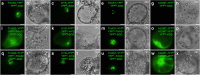
Plants produce an enormous array of biologically active metabolites, often with stereochemical variations on the same molecular scaffold. These changes in stereochemistry dramatically impact biological activity. Notably, the stereoisomers of the heteroyohimbine alkaloids show diverse pharmacological activities. We reported a medium chain dehydrogenase/reductase (MDR) from Catharanthus roseus that catalyses formation of a heteroyohimbine isomer. Here we report the discovery of additional heteroyohimbine synthases (HYSs), one of which produces a mixture of diastereomers. The crystal structures for three HYSs have been solved, providing insight into the mechanism of reactivity and stereoselectivity, with mutation of one loop transforming product specificity. Localization and gene silencing experiments provide a basis for understanding the function of these enzymes in vivo. This work sets the stage to explore how MDRs evolved to generate structural and biological diversity in specialized plant metabolism and opens the possibility for metabolic engineering of new compounds based on this scaffold.
Figures





References
-
- Shamma M. & Richey J. M. The stereochemistry of the heteroyohimbine alkaloids. J. Am. Chem. Soc. 85, 2507–2512 (1963).
-
- Allain H. & Bentue-Ferrer D. Clinical efficacy of almitrine-raubasine. An overview. Eur. Neurol. 39, 39–44 (1998). - PubMed
-
- Benzi G. Pharmacological features of an almitrine-raubasine combination. Activity at cerebral levels. Eur. Neurol. 39, 31–38 (1998). - PubMed
-
- Li S. et al. Assessment of the therapeutic activity of a combination of almitrine and raubasine on functional rehabilitation following ischaemic stroke. Curr. Med. Res. Opin. 20, 409–415 (2004). - PubMed
-
- Roquebert J. & Demichel P. Inhibition of the a1- and a2-adrenoceptor-mediated pressor response in pithed rats by raubasine, tetrahydroalstonine and akuammigine. Eur. J. Pharmacol. 106, 203–205 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

