Cost-Effectiveness of Primary HPV Testing, Cytology and Co-testing as Cervical Cancer Screening for Women Above Age 30 Years
- PMID: 27418345
- PMCID: PMC5071282
- DOI: 10.1007/s11606-016-3772-5
Cost-Effectiveness of Primary HPV Testing, Cytology and Co-testing as Cervical Cancer Screening for Women Above Age 30 Years
Abstract
Background: Cervical cancer screening guidelines for women aged ≥30 years allow for co-testing or primary cytology testing. Our objective was to determine the test characteristics and costs associated with Cytology, HPV and Co-testing screening strategies.
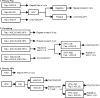
Main methods: Retrospective cohort study of women undergoing cervical cancer screening with both cytology and HPV (Hybrid Capture 2) testing from 2004 to 2010 in an integrated health system. The electronic health record was used to identify women aged ≥30 years who had co-testing. Unsatisfactory or unavailable test results and incorrectly ordered tests were excluded. The main outcome was biopsy-proven cervical intraepithelial neoplasia grade 3 or higher (CIN3+).
Key results: The final cohort consisted of 99,549 women. Subjects were mostly white (78.4 %), married (70.7 %), never smokers (61.3 %) and with private insurance (86.1 %). Overall, 5121 (5.1 %) tested positive for HPV and 6115 (6.1 %) had cytology ≥ ASCUS; 1681 had both and underwent colposcopy and 310 (0.3 %) had CIN3+. Sensitivity for CIN3+ was 91.9 % for Primary Cytology, 99.4 % for Co-testing, and 94.8 % for Primary HPV; specificity was 97.3 % for Co-testing and Primary Cytology and 97.9 % for Primary HPV. Over a 3-year screening interval, Primary HPV detected more cases of CIN3+ and was less expensive than Primary Cytology. Co-testing detected 14 more cases of CIN3+ than Primary HPV, but required an additional 100,277 cytology tests and 566 colposcopies at an added cost of $2.38 million, or $170,096 per additional case detected.
Conclusions: Primary HPV was more effective and less expensive than Primary Cytology. Primary HPV screening appears to represent a cost-effective alternative to Co-testing.
Conflict of interest statement
Compliance with Ethical Standards Funding/Support This study was funded internally by the Cleveland Clinic Research Program Committees, award number 2010-1050-R1. Conflict of Interest Xian Wen Jin MD, PhD: Speaking Bureau for Qiagen and Merck. Jerome Belinson MD: Support in kind (reagents and testing) and funds for direct support and research, under the auspices of Preventive Oncology International Inc., from Hologic Inc., Qiagen, Gen-Probe, Merck Inc., BGI Shenzhen, and GE Healthcare. None of the support described above was for the research described in this manuscript. All remaining authors declare that they do not have a conflict of interest.
Figures
Comment in
-
Capsule Commentary on Jin et. al., Cost-Effectiveness of Primary HPV Testing, Cytology and Co-testing as Cervical Cancer Screening for Women above Age 30 Years.J Gen Intern Med. 2016 Nov;31(11):1358. doi: 10.1007/s11606-016-3796-x. J Gen Intern Med. 2016. PMID: 27384535 Free PMC article. No abstract available.
Similar articles
-
Detection Rate of High-Grade Cervical Neoplasia and Cost-Effectiveness of High-Risk Human Papillomavirus Genotyping with Reflex Liquid-based Cytology in Cervical Cancer Screening.Ann Acad Med Singap. 2017 Jul;46(7):267-273. Ann Acad Med Singap. 2017. PMID: 28821890
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.Health Technol Assess. 2009 Nov;13(51):1-150, iii-iv. doi: 10.3310/hta13510. Health Technol Assess. 2009. PMID: 19891902 Clinical Trial.
-
Optimal Cervical Cancer Screening in Women Vaccinated Against Human Papillomavirus.J Natl Cancer Inst. 2016 Oct 18;109(2):djw216. doi: 10.1093/jnci/djw216. Print 2017 Feb. J Natl Cancer Inst. 2016. PMID: 27754955 Free PMC article.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. Report No.: 11-05157-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. Report No.: 11-05157-EF-1. PMID: 22553886 Free Books & Documents. Review.
Cited by
-
How Can a High-Performance Screening Strategy Be Determined for Cervical Cancer Prevention? Evidence From a Hierarchical Clustering Analysis of a Multicentric Clinical Study.Front Oncol. 2022 Jan 27;12:816789. doi: 10.3389/fonc.2022.816789. eCollection 2022. Front Oncol. 2022. PMID: 35155253 Free PMC article.
-
La pandémie et le dépistage du cancer du col: Est-ce enfin le temps d’adopter les tests d’autoprélèvement pour la détection du VPH au Canada?Can Fam Physician. 2022 Feb;68(2):99-102. doi: 10.46747/cfp.680299. Can Fam Physician. 2022. PMID: 35177497 Free PMC article. French. No abstract available.
-
Estimated Quality of Life and Economic Outcomes Associated With 12 Cervical Cancer Screening Strategies: A Cost-effectiveness Analysis.JAMA Intern Med. 2019 Jul 1;179(7):867-878. doi: 10.1001/jamainternmed.2019.0299. JAMA Intern Med. 2019. PMID: 31081851 Free PMC article.
-
Accuracy of cytological examination of Tao brush endometrial sampling in diagnosing endometrial premalignancy and malignancy.Int J Gynaecol Obstet. 2022 Dec;159(3):615-621. doi: 10.1002/ijgo.14204. Epub 2022 Apr 25. Int J Gynaecol Obstet. 2022. PMID: 35365908 Free PMC article.
-
The pandemic and cervical cancer screening: Is it finally time to adopt HPV self-swabbing tests in Canada?Can Fam Physician. 2022 Feb;68(2):90-92. doi: 10.46747/cfp.680290. Can Fam Physician. 2022. PMID: 35177494 Free PMC article. No abstract available.
References
-
- International Agency for Research on Cancer GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 31 May 2016.
-
- National Cancer Institute. SEER cancer statistics review, 1975–2009 (vintage 2009 populations). Available at: http://seer.cancer.gov/csr/1975_2009_pops09. Accessed May 31, 2016.
-
- American Cancer Society. Cancer Facts & Figures 2015. 2015.
-
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–72. doi: 10.3322/caac.21139. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical