Endotoxemia-menace, marker, or mistake?
- PMID: 27418356
- PMCID: PMC5014740
- DOI: 10.1189/jlb.3RU0316-151R
Endotoxemia-menace, marker, or mistake?
Abstract
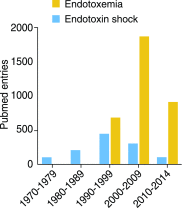
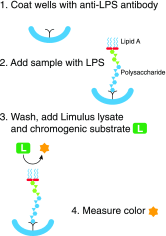
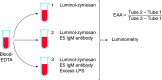
Endotoxemia is in its scientific ascendancy. Never has blood-borne, Gram-negative bacterial endotoxin (LPS) been invoked in the pathogenesis of so many diseases-not only as a trigger for septic shock, once its most cited role, but also as a contributor to atherosclerosis, obesity, chronic fatigue, metabolic syndrome, and many other conditions. Finding elevated plasma endotoxin levels has been essential supporting evidence for each of these links, yet the assays used to detect and quantitate endotoxin have important limitations. This article describes several assays for endotoxin in plasma, reviews what they do and do not measure, and discusses why LPS heterogeneity, LPS trafficking pathways, and host LPS inactivation mechanisms should be considered when interpreting endotoxin assay results.
Keywords: LPS; Limulus; endotoxin; lipopolysaccharide; translocation.
© Society for Leukocyte Biology.
Figures

References
-
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. (1989) The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 321, 280–287. - PubMed
-
- Marshall J. C. (2005) Lipopolysaccharide: an endotoxin or an exogenous hormone? Clin. Infect. Dis. 41 (Suppl 7), S470–S480. - PubMed
-
- Opal S. M., Scannon P. J., Vincent J. L., White M., Carroll S. F., Palardy J. E., Parejo N. A., Pribble J. P., Lemke J. H. (1999) Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180, 1584–1589. - PubMed
-
- Shands J. W. Jr.,Chun P. W. (1980) The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J. Biol. Chem. 255, 1221–1226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

