Severe muscle wasting and denervation in mice lacking the RNA-binding protein ZFP106
- PMID: 27418600
- PMCID: PMC4978283
- DOI: 10.1073/pnas.1608423113
Severe muscle wasting and denervation in mice lacking the RNA-binding protein ZFP106
Abstract
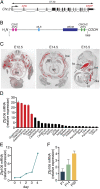
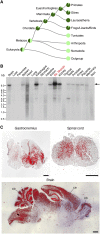
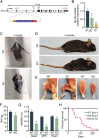
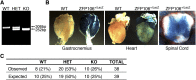
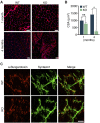
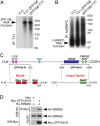
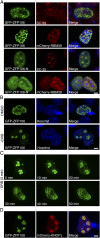
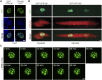
Innervation of skeletal muscle by motor neurons occurs through the neuromuscular junction, a cholinergic synapse essential for normal muscle growth and function. Defects in nerve-muscle signaling cause a variety of neuromuscular disorders with features of ataxia, paralysis, skeletal muscle wasting, and degeneration. Here we show that the nuclear zinc finger protein ZFP106 is highly enriched in skeletal muscle and is required for postnatal maintenance of myofiber innervation by motor neurons. Genetic disruption of Zfp106 in mice results in progressive ataxia and hindlimb paralysis associated with motor neuron degeneration, severe muscle wasting, and premature death by 6 mo of age. We show that ZFP106 is an RNA-binding protein that associates with the core splicing factor RNA binding motif protein 39 (RBM39) and localizes to nuclear speckles adjacent to spliceosomes. Upon inhibition of pre-mRNA synthesis, ZFP106 translocates with other splicing factors to the nucleolus. Muscle and spinal cord of Zfp106 knockout mice displayed a gene expression signature of neuromuscular degeneration. Strikingly, altered splicing of the Nogo (Rtn4) gene locus in skeletal muscle of Zfp106 knockout mice resulted in ectopic expression of NOGO-A, the neurite outgrowth factor that inhibits nerve regeneration and destabilizes neuromuscular junctions. These findings reveal a central role for Zfp106 in the maintenance of nerve-muscle signaling, and highlight the involvement of aberrant RNA processing in neuromuscular disease pathogenesis.
Keywords: ZNF106; amyotrophic lateral sclerosis (ALS); motor neuron disease (MND); neuromuscular junction (NMJ); pre-mRNA splicing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hirsch NP. Neuromuscular junction in health and disease. Br J Anaesth. 2007;99(1):132–138. - PubMed
-
- Goulet BB, Kothary R, Parks RJ. At the “junction” of spinal muscular atrophy pathogenesis: The role of neuromuscular junction dysfunction in SMA disease progression. Curr Mol Med. 2013;13(7):1160–1174. - PubMed
-
- Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015;95(3):809–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

