Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication
- PMID: 27418644
- PMCID: PMC5034739
- DOI: 10.1182/blood-2016-03-707547
Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication
Abstract
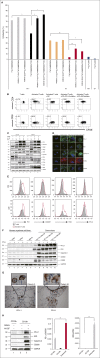
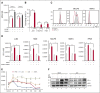
The number and activity of osteoclasts (OCs) are strongly enhanced by myeloma cells, leading to significant bone lesions in patients with multiple myeloma (MM). Mechanisms remain elusive as to whether myeloma-supporting OCs also induce suppressive immune bone marrow (BM) microenvironment. Here, we first show that OCs significantly protect MM cells against T-cell-mediated cytotoxicity via direct inhibition of proliferating CD4(+) and CD8(+) T cells. The immune checkpoint molecules programmed death ligand 1 (PD-L1), Galectin-9, herpesvirus entry mediator (HVEM), and CD200, as well as T-cell metabolism regulators indoleamine 2, 3-dioxygenase (IDO), and CD38 are significantly upregulated during osteoclastogenesis. Importantly, the levels of these molecules, except CD38, are higher in OCs than in MM cells. Anti-PD-L1 monoclonal antibody (mAb) and IDO inhibitor partly overcome OC-inhibited T-cell responses against MM cells, confirming their roles in OC-suppressed MM cell lysis by cytotoxic T cells. In addition, Galectin-9 and a proliferation-induced ligand (APRIL), secreted by OCs, are significantly upregulated during osteoclastogenesis. Galectin-9 specifically induces apoptosis of T cells while sparing monocytes and MM cells. APRIL induces PD-L1 expression in MM cells, providing additional immune inhibition by OCs. Moreover, CD38 is significantly upregulated during osteoclastogenesis. When targeted by an anti-CD38 mAb, suppressive T-cell function by OCs is alleviated, associated with downregulation of HVEM and IDO. Taken together, these results define the expression of multiple immune proteins and cytokines in OCs essential for suppressive MM BM milieu. These results further support the combination of targeting these molecules to improve anti-MM immunity.
© 2016 by The American Society of Hematology.
Figures





References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. - PubMed
-
- Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17(6):1278–1286. - PubMed
-
- Wu Y, Humphrey MB, Nakamura MC. Osteoclasts - the innate immune cells of the bone. Autoimmunity. 2008;41(3):183–194. - PubMed
-
- Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5(12):667–676. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

