Effects of anti-vascular endothelial growth factor monoclonal antibody (bevacizumab) on lens epithelial cells
- PMID: 27418802
- PMCID: PMC4935105
- DOI: 10.2147/OPTH.S103443
Effects of anti-vascular endothelial growth factor monoclonal antibody (bevacizumab) on lens epithelial cells
Abstract
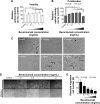
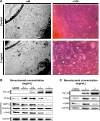
The molecular and cellular effects of anti-vascular endothelial growth factor monoclonal antibody (bevacizumab) on lens epithelial cells (LECs) were examined using both an immortalized human lens epithelial cell line and a porcine capsular bag model. After treatment with various concentrations of bevacizumab, cell viability and proliferation patterns were evaluated using the water-soluble tetrazolium salt assay and 5-bromo-2'-deoxyuridine enzyme-linked immunosorbent assay, respectively. The scratch assay and Western blot analysis were employed to validate the cell migration pattern and altered expression levels of signaling molecules related to the epithelial-mesenchymal transition (EMT). Application of bevacizumab induced a range of altered cellular events in a concentration-dependent manner. A 0.1-2 mg/mL concentration demonstrated dose-dependent increase in proliferation and viability of LECs. However, 4 mg/mL decreased cell proliferation and viability. Cell migrations displayed dose-dependent retardation from 0.1 mg/mL bevacizumab treatment. Transforming growth factor-β2 expression was markedly increased in a dose-dependent manner, and α-smooth muscle actin, matrix metalloproteinase-9, and vimentin expression levels showed dose-dependent changes in a B3 cell line. Microscopic observation of porcine capsular bag revealed changes in cellular morphology and a decline in cell density compared to the control after 2 mg/mL treatment. The central aspect of posterior capsule showed delayed confluence, and the factors related to EMT revealed similar expression patterns to those identified in the cell line. Based on these results, bevacizumab modulates the proliferation and viability of LECs and induces morphological alterations through the modulation of expression patterns of specific factors related to the EMT.
Keywords: avastin; bevacizumab; lens epithelial cell; transforming growth factor; vascular endothelial growth factor.
Figures

References
-
- Shui YB, Wang X, Hu JS, et al. Vascular endothelial growth factor expression and signaling in the lens. Invest Ophthalmol Vis Sci. 2003;44(9):3911–3919. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

