Efficacy and safety of ipratropium bromide/salbutamol sulphate administered in a hydrofluoroalkane metered-dose inhaler for the treatment of COPD
- PMID: 27418820
- PMCID: PMC4934560
- DOI: 10.2147/COPD.S89923
Efficacy and safety of ipratropium bromide/salbutamol sulphate administered in a hydrofluoroalkane metered-dose inhaler for the treatment of COPD
Abstract
Background: The use of chlorofluorocarbons (CFCs) has contributed to the depletion of the stratospheric ozone layer resulting in serious health concerns. Ipratropium bromide/salbutamol sulphate CFC-pressurized metered-dose inhalers (IB/SAL-CFC pMDI) have been in widespread use for many years without any apparent ill consequences. This combination has now been reformulated using the hydrofluoroalkane (HFA) propellant. This study sought to establish the clinical noninferiority of a new HFA-containing IB/SAL pMDI to the conventional IB/SAL-CFC pMDI in subjects with mild/moderate COPD.
Methods: This was a randomized, double-blind, parallel-group, multicenter study in two consecutive periods: a 14-day run-in period followed by a 85-day treatment period. Eligible mild-to-moderate stable COPD subjects aged 40-75 years were enrolled into the study and entered the run-in period during which subjects withdrew all the bronchodilators, except for salbutamol as rescue medication. Subjects were randomized to 85 days treatment with either IB/SAL-HFA or IB/SAL-CFC, 20 μg qid.
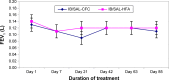
Results: Of the 290 randomized patients, 249 completed the study. The primary efficacy variable was the change in forced expiratory volume in one second from predose to 60 minutes after dosing on day 85. At the end of the treatment period, the adjusted mean change in forced expiratory volume in one second at 60 minutes was 123 mL in the IB/SAL-HFA pMDI group and 115 mL in the IB/SAL-CFC pMDI group. Because the lower limit of the 95% confidence interval for the between-group difference (-62 mL) was well within the noninferiority margin (-100 mL), the HFA formulation was deemed clinically noninferior to the CFC formulation. This finding was supported by secondary efficacy assessments. Both formulations of IB/SAL were well tolerated during the prolonged multiple dosing.
Conclusion: It is concluded that IB/SAL-HFA pMDI provides effective bronchodilation of similar degree to that achieved with IB/SAL-CFC pMDI. Therefore, IB/SAL-HFA pMDI is a valuable alternative to IB/SAL-CFC pMDI.
Keywords: COPD; FEV1; hydrofluoroalkane; ipratropium/salbutamol; noninferiority; pressurized metered-dose inhaler.
Figures
Similar articles
-
Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered-dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double-blind, placebo-controlled, 12-week study.Clin Ther. 2006 Jan;28(1):73-85. doi: 10.1016/j.clinthera.2006.01.008. Clin Ther. 2006. PMID: 16490581 Clinical Trial.
-
Double-blind, double-dummy, multinational, multicenter, parallel-group design clinical trial of clinical non-inferiority of formoterol 12 microg/unit dose in a b.i.d. regimen administered via an HFA-propellant-pMDI or a dry powder inhaler in a 12-week treatment period of moderate to severe stable persistent asthma in adult patients.Respiration. 2005;72 Suppl 1:20-7. doi: 10.1159/000083689. Respiration. 2005. PMID: 15915009 Clinical Trial.
-
Equivalence of salbutamol 200 microg four times daily propelled by propellants 11 and 12 or HFA 134a in mild to moderate asthmatics. Eastern European study group.Respir Med. 2000 Jun;94 Suppl B:S22-8. doi: 10.1016/s0954-6111(00)90150-1. Respir Med. 2000. PMID: 10919682 Clinical Trial.
-
A review of ipratropium bromide/fenoterol hydrobromide (Berodual) delivered via Respimat Soft Mist Inhaler in patients with asthma and chronic obstructive pulmonary disease.Drugs. 2004;64(15):1671-82. doi: 10.2165/00003495-200464150-00005. Drugs. 2004. PMID: 15257628 Review.
-
Ipratropium bromide HFA.Treat Respir Med. 2005;4(3):215-20; discussion 221-2. doi: 10.2165/00151829-200504030-00006. Treat Respir Med. 2005. PMID: 15987237 Review.
Cited by
-
Ipratropium bromide and noninvasive ventilation treatment for COPD.Am J Transl Res. 2022 May 15;14(5):3319-3326. eCollection 2022. Am J Transl Res. 2022. PMID: 35702113 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [homepage on the Internet] Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease [updated 2015] [Accessed September 9, 2015]. Available from: http://www.goldcopd.org.
-
- National Institute for Clinical Excellence . Chronic Obstructive Pulmonary Disease Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care Clinical Guideline 12. London: National Institute for Clinical Excellence; 2004.
-
- American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. - PubMed
-
- COMBIVENT Inhalation Aerosol Study Group In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. Chest. 1994;105:1411–1419. - PubMed
-
- CPMP/391/97 . Consolidation of CPMP Reviews of Alternatives to Chlorofluorocarbon Propellants. London: 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

