Interactions between Dorsal and Ventral Root Stimulation on the Generation of Locomotor-Like Activity in the Neonatal Mouse Spinal Cord
- PMID: 27419215
- PMCID: PMC4937207
- DOI: 10.1523/ENEURO.0101-16.2016
Interactions between Dorsal and Ventral Root Stimulation on the Generation of Locomotor-Like Activity in the Neonatal Mouse Spinal Cord
Abstract
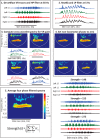
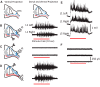
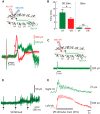
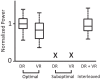
We investigated whether dorsal (DR) and ventral root (VR) stimulus trains engage common postsynaptic components to activate the central pattern generator (CPG) for locomotion in the neonatal mouse spinal cord. VR stimulation did not activate the first order interneurons mediating the activation of the locomotor CPG by sacrocaudal afferent stimulation. Simultaneous stimulation of adjacent dorsal or ventral root pairs, subthreshold for evoking locomotor-like activity, did not summate to activate the CPG. This suggests that locomotor-like activity is triggered when a critical class of efferent or afferent axons is stimulated and does not depend on the number of stimulated axons or activated postsynaptic neurons. DR- and VR-evoked episodes exhibited differences in the coupling between VR pairs. In DR-evoked episodes, the coupling between the ipsilateral and contralateral flexor/extensor roots was similar and stronger than the bilateral extensor roots. In VR-evoked episodes, ipsilateral flexor/extensor coupling was stronger than both the contralateral flexor/extensor and the bilateral extensor coupling. For both types of stimulation, the coupling was greatest between the bilateral L1/L2 flexor-dominated roots. This indicates that the recruitment and/or the firing pattern of motoneurons differed in DR and VR-evoked episodes. However, the DR and VR trains do not appear to activate distinct CPGs because trains of DR and VR stimuli at frequencies too low to evoke locomotor-like activity did so when they were interleaved. These results indicate that the excitatory actions of VR stimulation converge onto the CPG through an unknown pathway that is not captured by current models of the locomotor CPG.
Keywords: central pattern generator; dorsal root; locomotion; spinal cord; ventral root.
Conflict of interest statement
The authors report no conflict of interest.
Figures






Similar articles
-
Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization.J Physiol. 2012 Oct 1;590(19):4735-59. doi: 10.1113/jphysiol.2012.240895. Epub 2012 Aug 6. J Physiol. 2012. PMID: 22869012 Free PMC article.
-
Antidromic discharges of dorsal root afferents and inhibition of the lumbar monosynaptic reflex in the neonatal rat.Neuroscience. 1999 Apr;90(1):165-76. doi: 10.1016/s0306-4522(98)00435-7. Neuroscience. 1999. PMID: 10188943
-
The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres.J Physiol. 2004 Jul 1;558(Pt 1):213-24. doi: 10.1113/jphysiol.2004.064717. Epub 2004 Apr 30. J Physiol. 2004. PMID: 15121804 Free PMC article.
-
The sacral networks and neural pathways used to elicit lumbar motor rhythm in the rodent spinal cord.Front Neural Circuits. 2014 Dec 3;8:143. doi: 10.3389/fncir.2014.00143. eCollection 2014. Front Neural Circuits. 2014. PMID: 25520624 Free PMC article. Review.
-
Formation of the central pattern generator for locomotion in the rat and mouse.Brain Res Bull. 2000 Nov 15;53(5):661-9. doi: 10.1016/s0361-9230(00)00399-3. Brain Res Bull. 2000. PMID: 11165801 Review.
Cited by
-
Generation of locomotor‑like activity using monopolar intraspinal electrical microstimulation in rats.Exp Ther Med. 2023 Oct 18;26(6):560. doi: 10.3892/etm.2023.12259. eCollection 2023 Dec. Exp Ther Med. 2023. PMID: 37941590 Free PMC article.
-
Shared Components of Rhythm Generation for Locomotion and Scratching Exist Prior to Motoneurons.Front Neural Circuits. 2017 Aug 11;11:54. doi: 10.3389/fncir.2017.00054. eCollection 2017. Front Neural Circuits. 2017. PMID: 28848402 Free PMC article.
-
Ventral root evoked entrainment of disinhibited bursts across early postnatal development in mice.IBRO Rep. 2020 Oct 27;9:310-318. doi: 10.1016/j.ibror.2020.10.005. eCollection 2020 Dec. IBRO Rep. 2020. PMID: 33294722 Free PMC article.
-
V1 interneurons regulate the pattern and frequency of locomotor-like activity in the neonatal mouse spinal cord.PLoS Biol. 2019 Sep 12;17(9):e3000447. doi: 10.1371/journal.pbio.3000447. eCollection 2019 Sep. PLoS Biol. 2019. PMID: 31513565 Free PMC article.
-
Resetting the Respiratory Rhythm with a Spinal Central Pattern Generator.eNeuro. 2019 May 1;6(2):ENEURO.0116-19.2019. doi: 10.1523/ENEURO.0116-19.2019. Print 2019 Mar/Apr. eNeuro. 2019. PMID: 31043462 Free PMC article.
References
-
- Atsuta Y, Garcia-Rill E, Skinner RD (1990) Characteristics of electrically induced locomotion in rat in vitro brain stem-spinal cord preparation. J Neurophysiol 64:727– 735. - PubMed
-
- Biscoe TJ, Nickels SM, Stirling CA (1982) Numbers and sizes of nerve fibres in mouse spinal roots. Q J Exp Physiol 67:473–494. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
