Cell death is not essential for caspase-1-mediated interleukin-1β activation and secretion
- PMID: 27419363
- PMCID: PMC5071572
- DOI: 10.1038/cdd.2016.69
Cell death is not essential for caspase-1-mediated interleukin-1β activation and secretion
Abstract
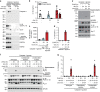
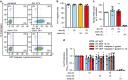
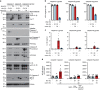
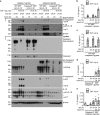
Caspase-1 cleaves and activates the pro-inflammatory cytokine interleukin-1 beta (IL-1β), yet the mechanism of IL-1β release and its dependence on cell death remains controversial. To address this issue, we generated a novel inflammasome independent system in which we directly activate caspase-1 by dimerization. In this system, caspase-1 dimerization induced the cleavage and secretion of IL-1β, which did not require processing of caspase-1 into its p20 and p10 subunits. Moreover, direct caspase-1 dimerization allowed caspase-1 activation of IL-1β to be separated from cell death. Specifically, we demonstrate at the single cell level that IL-1β can be released from live, metabolically active, cells following caspase-1 activation. In addition, we show that dimerized or endogenous caspase-8 can also directly cleave IL-1β into its biologically active form, in the absence of canonical inflammasome components. Therefore, cell death is not obligatory for the robust secretion of bioactive IL-1β.
Figures


References
-
- Vince JE. When beauty is skin deep: regulation of the wound response by caspase-8, RIPK3, and the inflammasome. J Invest Dermatol 2015; 135: 1936–1939. - PubMed
-
- Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim Biophys Acta 2014; 1840: 1433–1440. - PubMed
-
- Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 2015; 74: 213–218. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

