Construction of an immunotoxin, HN3-mPE24, targeting glypican-3 for liver cancer therapy
- PMID: 27419635
- PMCID: PMC5464801
- DOI: 10.18632/oncotarget.10592
Construction of an immunotoxin, HN3-mPE24, targeting glypican-3 for liver cancer therapy
Abstract
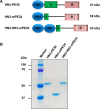
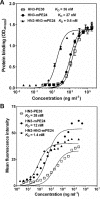
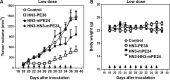
Glypican-3 (GPC3) is overexpressed in hepatocellular carcinoma (HCC). We constructed a recombinant immunotoxin, HN3-mPE24, which contains a truncated form of Pseudomonas exotoxin A. The toxin portion lacks most of domain II and has seven point mutations in domain III to remove the B-cell epitopes thought to be responsible for causing off-target side effects and immunogenicity. We also fused a bivalent HN3 to mPE24. We tested these two molecules for GPC3 binding and cytotoxicity in HCC cell models. The KD values of HN3-mPE24 and HN3-HN3-mPE24 for GPC3-expressing tumor cells were 12 nM and 1.4 nM, respectively. The IC50 values of HN3-mPE24 and HN3-HN3-mPE24 for HCC cells were 0.2 nM and 0.4 nM, respectively. We also evaluated their toxicity and anti-tumor efficacy in mice. The maximum tolerated doses of HN3-mPE24 and HN3-HN3-mPE24 were 7 mg kg-1 and 3.6 mg kg-1, respectively. We treated mice with 5 mg kg-1 of HN3-mPE24 intravenously every other day for ten injections. The alpha-fetoprotein level of HN3-mPE24 treated group was approximately 700 fold less than that of the untreated group (1.1 μg ml-1 vs. 692.1 μg ml-1). In addition, 25% of the mice treated with HN3-mPE24 survived to the end of this study, which was 105 days after HCC tumor implantation. In conclusion, the HN3-mPE24 immunotoxin caused liver tumor regressions and extended survival with no significant side effects in mice. It is a promising candidate for the treatment of liver cancer that may be readily translated to humans.
Keywords: glypican-3 or GPC3; hepatocellular carcinoma; mouse xenograft model; recombinant immunotoxin; single-domain antibody fragment.
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures



Similar articles
-
Engineered Anti-GPC3 Immunotoxin, HN3-ABD-T20, Produces Regression in Mouse Liver Cancer Xenografts Through Prolonged Serum Retention.Hepatology. 2020 May;71(5):1696-1711. doi: 10.1002/hep.30949. Epub 2020 Jan 27. Hepatology. 2020. PMID: 31520528 Free PMC article.
-
Glypican-3 Targeting Immunotoxins for the Treatment of Liver Cancer.Toxins (Basel). 2016 Sep 22;8(10):274. doi: 10.3390/toxins8100274. Toxins (Basel). 2016. PMID: 27669301 Free PMC article. Review.
-
Exploring Glypican-3 targeted CAR-NK treatment and potential therapy resistance in hepatocellular carcinoma.PLoS One. 2025 Jan 22;20(1):e0317401. doi: 10.1371/journal.pone.0317401. eCollection 2025. PLoS One. 2025. PMID: 39841705 Free PMC article.
-
Persistent Polyfunctional Chimeric Antigen Receptor T Cells That Target Glypican 3 Eliminate Orthotopic Hepatocellular Carcinomas in Mice.Gastroenterology. 2020 Jun;158(8):2250-2265.e20. doi: 10.1053/j.gastro.2020.02.011. Epub 2020 Feb 12. Gastroenterology. 2020. PMID: 32060001 Free PMC article.
-
Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment.Med Res Rev. 2018 Mar;38(2):741-767. doi: 10.1002/med.21455. Epub 2017 Jun 16. Med Res Rev. 2018. PMID: 28621802 Review.
Cited by
-
Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics.Front Oncol. 2020 Jul 23;10:1182. doi: 10.3389/fonc.2020.01182. eCollection 2020. Front Oncol. 2020. PMID: 32793488 Free PMC article. Review.
-
Immunotoxins Immunotherapy against Hepatocellular Carcinoma: A Promising Prospect.Toxins (Basel). 2021 Oct 11;13(10):719. doi: 10.3390/toxins13100719. Toxins (Basel). 2021. PMID: 34679012 Free PMC article. Review.
-
Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma.Front Oncol. 2022 Feb 16;12:824208. doi: 10.3389/fonc.2022.824208. eCollection 2022. Front Oncol. 2022. PMID: 35251989 Free PMC article. Review.
-
Glypican-3: A molecular marker for the detection and treatment of hepatocellular carcinoma☆.Liver Res. 2020 Dec;4(4):168-172. doi: 10.1016/j.livres.2020.11.003. Epub 2020 Nov 11. Liver Res. 2020. PMID: 33384879 Free PMC article.
-
Nanobodies: Robust miniprotein binders in biomedicine.Adv Drug Deliv Rev. 2023 Apr;195:114726. doi: 10.1016/j.addr.2023.114726. Epub 2023 Feb 7. Adv Drug Deliv Rev. 2023. PMID: 36754285 Free PMC article. Review.
References
-
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. - PubMed
-
- Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15:42–52. - PubMed
-
- Blechacz B, Mishra L. Hepatocellular carcinoma biology. Recent Results Cancer Res. 2013;190:1–20. - PubMed
-
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

