Bacterial Suppression of RNA Polymerase II-Dependent Host Gene Expression
- PMID: 27420101
- PMCID: PMC5039429
- DOI: 10.3390/pathogens5030049
Bacterial Suppression of RNA Polymerase II-Dependent Host Gene Expression
Abstract
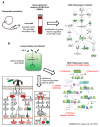
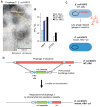
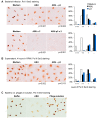
Asymptomatic bacteriuria (ABU) is a bacterial carrier state in the urinary tract that resembles commensalism at other mucosal sites. ABU strains often lack the virulence factors that characterize uropathogenic Escherichia coli (E. coli) strains and therefore elicit weak innate immune responses in the urinary tract. In addition, ABU strains are active modifiers of the host environment, which they influence by suppressing RNA polymerase II (Pol II)-dependent host gene expression. In patients inoculated with the ABU strain E. coli 83972, gene expression was markedly reduced after 24 h (>60% of all regulated genes). Specific repressors and activators of Pol II-dependent transcription were modified, and Pol II Serine 2 phosphorylation was significantly inhibited, indicating reduced activity of the polymerase. This active inhibition included disease-associated innate immune response pathways, defined by TLR4, IRF-3 and IRF-7, suggesting that ABU strains persist in human hosts by active suppression of the antibacterial defense. In a search for the mechanism of inhibition, we compared the whole genome sequences of E. coli 83972 and the uropathogenic strain E. coli CFT073. In addition to the known loss of virulence genes, we observed that the ABU strain has acquired several phages and identified the lytic Prophage 3 as a candidate Pol II inhibitor. Intact phage particles were released by ABU during in vitro growth in human urine. To address if Prophage 3 affects Pol II activity, we constructed a Prophage 3 negative deletion mutant in E. coli 83972 and compared the effect on Pol II phosphorylation between the mutant and the E. coli 83972 wild type (WT) strains. No difference was detected, suggesting that the Pol II inhibitor is not encoded by the phage. The review summarizes the evidence that the ABU strain E. coli 83972 modifies host gene expression by inhibition of Pol II phosphorylation, and discusses the ability of ABU strains to actively create an environment that enhances their persistence.
Keywords: RNA polymerase II; asymptomatic bacteriuria; gene expression; phages; transcriptional modulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zdziarski J., Brzuszkiewicz E., Wullt B., Liesegang H., Biran D., Voigt B., Gronberg-Hernandez J., Ragnarsdottir B., Hecker M., Ron E.Z., et al. Host imprints on bacterial genomes-rapid, divergent evolution in individual patients. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001078. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

