OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2 O2 signalling in rice
- PMID: 27420922
- PMCID: PMC5258865
- DOI: 10.1111/pbi.12601
OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2 O2 signalling in rice
Abstract
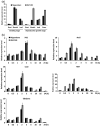
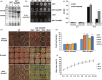
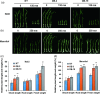
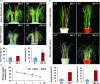
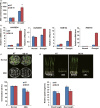
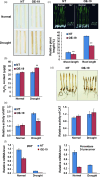
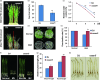
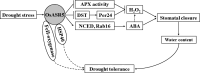
Drought is one of the major abiotic stresses that directly implicate plant growth and crop productivity. Although many genes in response to drought stress have been identified, genetic improvement to drought resistance especially in food crops is showing relatively slow progress worldwide. Here, we reported the isolation of abscisic acid, stress and ripening (ASR) genes from upland rice variety, IRAT109 (Oryza sativa L. ssp. japonica), and demonstrated that overexpression of OsASR5 enhanced osmotic tolerance in Escherichia coli and drought tolerance in Arabidopsis and rice by regulating leaf water status under drought stress conditions. Moreover, overexpression of OsASR5 in rice increased endogenous ABA level and showed hypersensitive to exogenous ABA treatment at both germination and postgermination stages. The production of H2 O2 , a second messenger for the induction of stomatal closure in response to ABA, was activated in overexpression plants under drought stress conditions, consequently, increased stomatal closure and decreased stomatal conductance. In contrast, the loss-of-function mutant, osasr5, showed sensitivity to drought stress with lower relative water content under drought stress conditions. Further studies demonstrated that OsASR5 functioned as chaperone-like protein and interacted with stress-related HSP40 and 2OG-Fe (II) oxygenase domain containing proteins in yeast and plants. Taken together, we suggest that OsASR5 plays multiple roles in response to drought stress by regulating ABA biosynthesis, promoting stomatal closure, as well as acting as chaperone-like protein that possibly prevents drought stress-related proteins from inactivation.
Keywords: ABA; Oryza sativa; OsASR5; Drought; stomata; water content.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures


Similar articles
-
The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression.BMC Plant Biol. 2016 Jul 13;16(1):158. doi: 10.1186/s12870-016-0845-x. BMC Plant Biol. 2016. PMID: 27411911 Free PMC article.
-
Roles of a maize phytochrome-interacting factors protein ZmPIF3 in regulation of drought stress responses by controlling stomatal closure in transgenic rice without yield penalty.Plant Mol Biol. 2018 Jul;97(4-5):311-323. doi: 10.1007/s11103-018-0739-4. Epub 2018 Jun 5. Plant Mol Biol. 2018. PMID: 29869742
-
ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis.PLoS One. 2015 Apr 17;10(4):e0125168. doi: 10.1371/journal.pone.0125168. eCollection 2015. PLoS One. 2015. PMID: 25886365 Free PMC article.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
-
Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought.Int J Mol Sci. 2022 Nov 26;23(23):14824. doi: 10.3390/ijms232314824. Int J Mol Sci. 2022. PMID: 36499153 Free PMC article. Review.
Cited by
-
Amino acid motifs for the identification of novel protein interactants.Comput Struct Biotechnol J. 2022 Dec 10;21:326-334. doi: 10.1016/j.csbj.2022.12.012. eCollection 2023. Comput Struct Biotechnol J. 2022. PMID: 36582434 Free PMC article. Review.
-
Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives.Front Plant Sci. 2017 Jul 11;8:1214. doi: 10.3389/fpls.2017.01214. eCollection 2017. Front Plant Sci. 2017. PMID: 28744298 Free PMC article. Review.
-
Profiling of Key Hub Genes Using a Two-State Weighted Gene Co-Expression Network of 'Jao Khao' Rice under Soil Salinity Stress Based on Time-Series Transcriptome Data.Int J Mol Sci. 2024 Oct 16;25(20):11086. doi: 10.3390/ijms252011086. Int J Mol Sci. 2024. PMID: 39456877 Free PMC article.
-
AtTLP2, a Tubby-like protein, plays intricate roles in abiotic stress signalling.Plant Cell Rep. 2023 Feb;42(2):235-252. doi: 10.1007/s00299-022-02953-z. Epub 2022 Nov 28. Plant Cell Rep. 2023. PMID: 36437308
-
ThASR3 confers salt and osmotic stress tolerances in transgenic Tamarix and Arabidopsis.BMC Plant Biol. 2022 Dec 14;22(1):586. doi: 10.1186/s12870-022-03942-w. BMC Plant Biol. 2022. PMID: 36517747 Free PMC article.
References
-
- Cho, E.K. and Hong, C.B. (2006) Over‐expression of tobacco NtHSP70‐1 contributes to drought‐stress tolerance in plants. Plant Cell Rep. 25, 349–358. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

