Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial
- PMID: 27421268
- PMCID: PMC4946239
- DOI: 10.1186/s12874-016-0180-9
Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial
Abstract
Background: A systematic review on the use of incentives to promote questionnaire return in clinical trials suggest they are effective, but not all studies have sufficient funds to use them. Promising an incentive once data are returned can reduce the cost-burden of this approach, with possible further cost-savings if the offer were restricted to reminder letters only. This study aimed to evaluate the effect of promising a monetary incentive at first mailout versus a promise on reminder letters only.
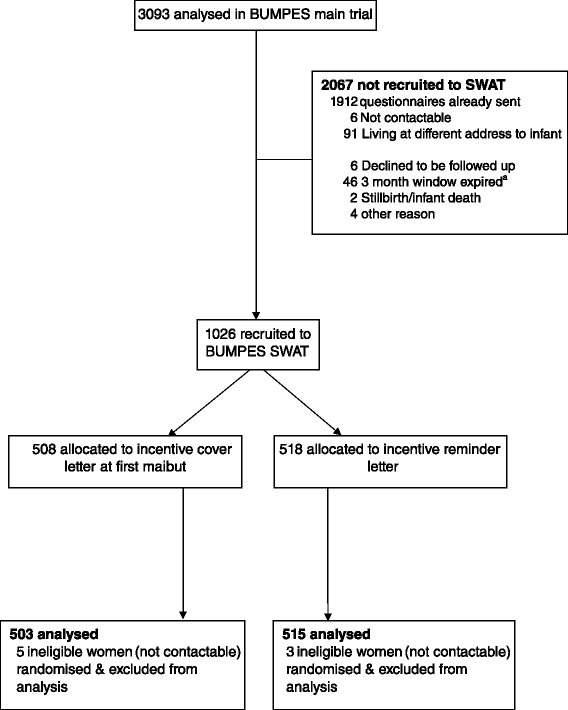
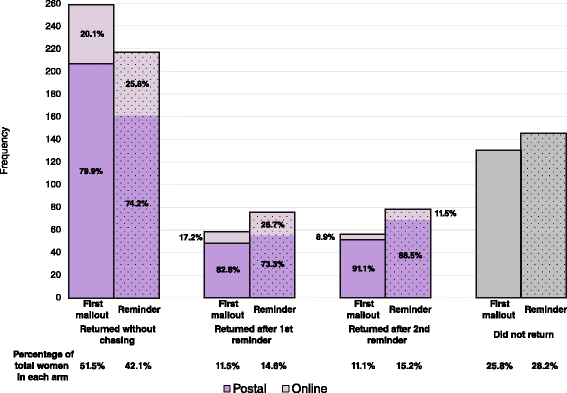
Methods: This was a randomised Study Within A Trial (SWAT) nested within BUMPES, a multicentre randomised controlled trial of maternal position in the late stage of labour in women with an epidural. The follow-up questionnaire asked for information on the women's health, wellbeing and health service use one year following the birth of their baby. Women who consented to be contacted were randomised to a promise of a monetary incentive at first mailout or a promise on reminder letters only. Women were given an option of completing the questionnaire on paper or on online. The incentive was posted out on receipt of a completed questionnaire. The primary outcome was the overall return rate, and secondary outcomes were the return rate without any chasing from the study office, and the total cost of the vouchers.
Results: A total of 1,029 women were randomised, 508 to the first mailout group and 518 to the reminder group. There was no evidence to suggest a difference between groups in the overall return rate (adjusted RR 1.03 (95 % CI 0.96 to 1.11), however the proportion returned without chasing was higher in the first mailout group (adjusted RR 1.22, 95 % CI 1.07 to 1.39). The total cost of the vouchers per participant was higher in the first mailout group (mean difference £4.56, 95 % CI £4.02 to £5.11).
Conclusions: Offering a monetary incentive when a reminder is required could be cost-effective depending on the sample size of the study and the resources available to administer the reminder letters.
Trial registration: The BUMPES Trial is registered with Current Controlled Trials: ISRCTN35706297 , 26(th) August 2009.
Keywords: BUMPES; Clinical trial; Follow-up; Incentive; Questionnaires; Randomised; Return rates; SWAT.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources