Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke
- PMID: 27422020
- PMCID: PMC4947279
- DOI: 10.1186/s12868-016-0284-5
Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke
Abstract
Background: Perivascular stromal cells (PSCs) are a recently identified cell type that comprises a small percentage of the platelet derived growth factor receptor-β+ cells within the CNS perivascular space. PSCs are activated following injury to the brain or spinal cord, expand in number and contribute to fibrotic scar formation within the injury site. Beyond fibrosis, their high density in the lesion core makes them a potential significant source of signals that act on neural cells adjacent to the lesion site.
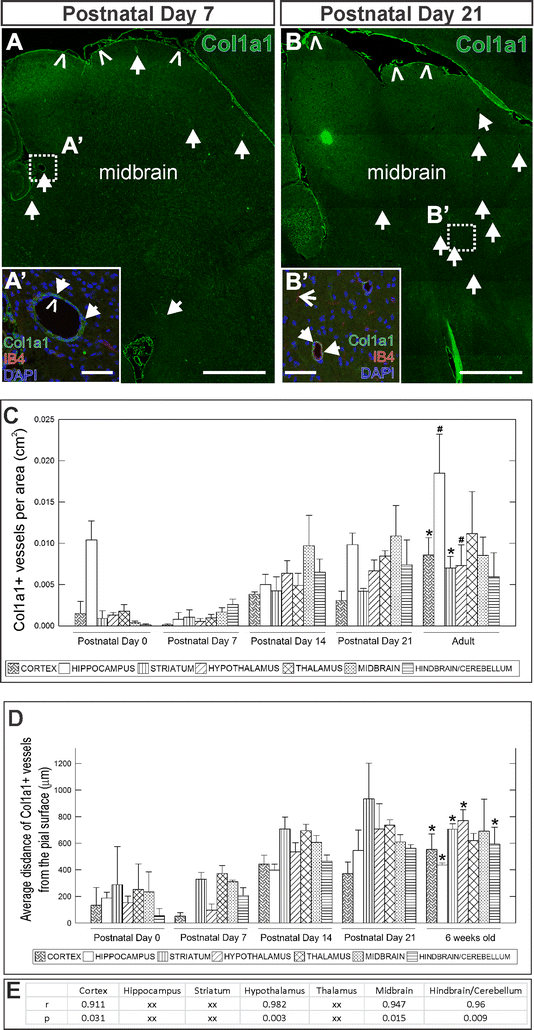
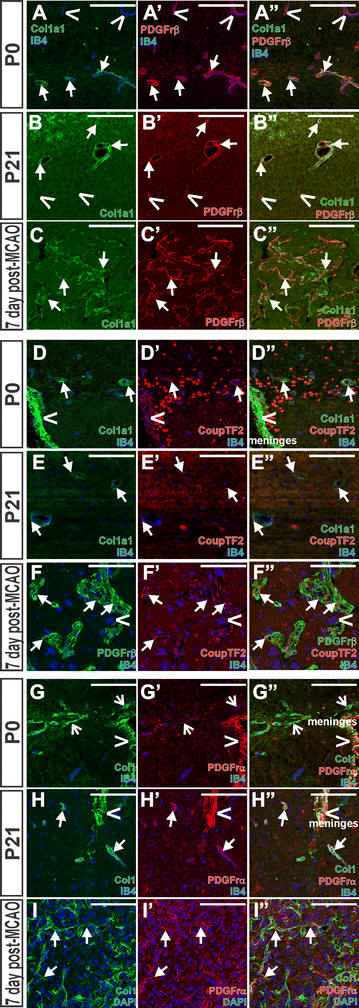
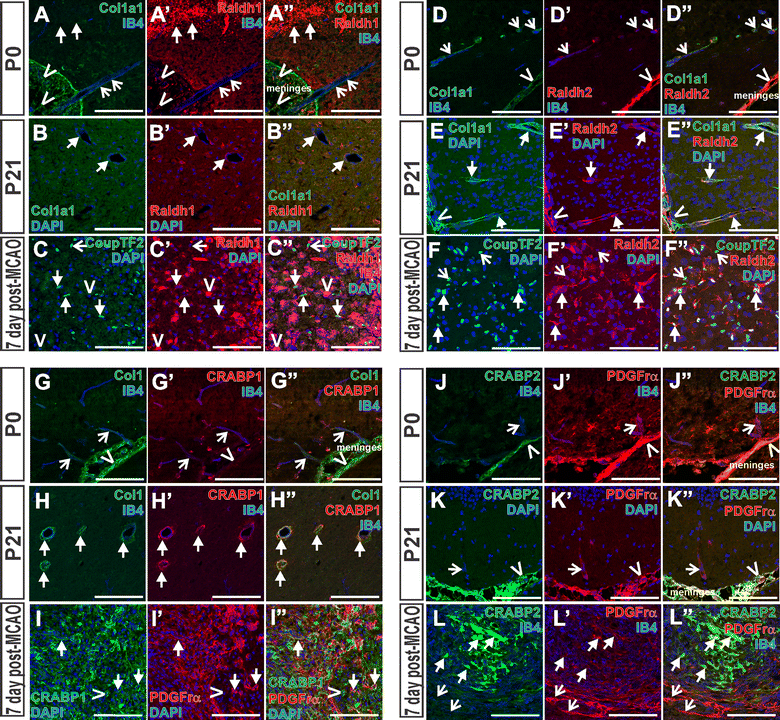
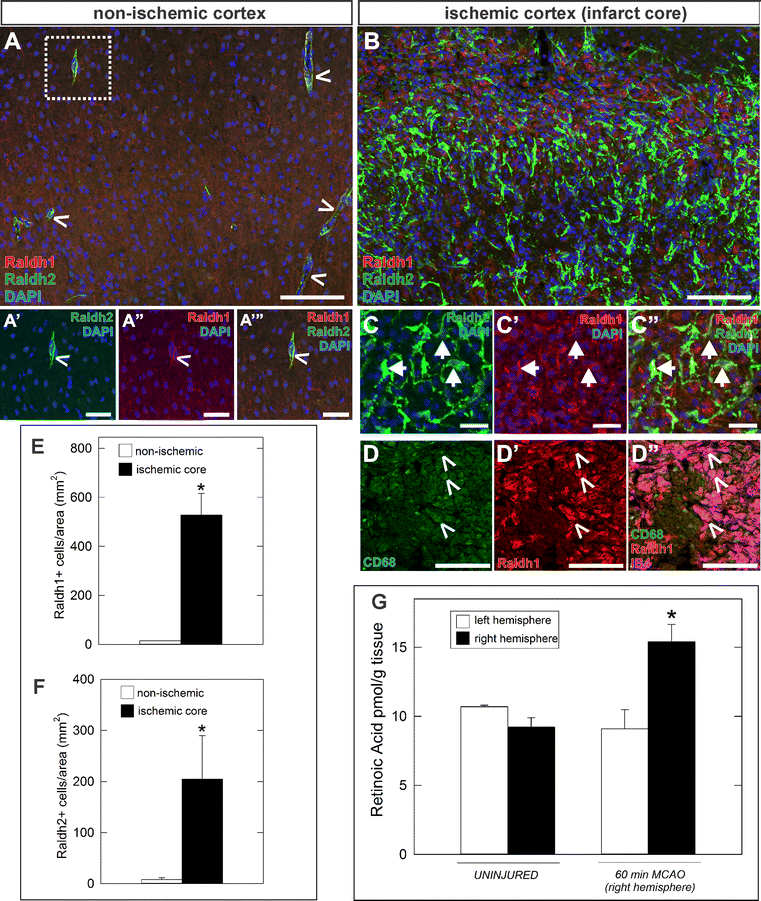
Results: Our developmental analysis of PSCs, defined by expression of Collagen1a1 in the maturing brain, revealed that PSCs first appear postnatally and may originate from the meninges. PSCs express many of the same markers as meningeal fibroblasts, including expression of the retinoic acid (RA) synthesis proteins Raldh1 and Raldh2. Using a focal brain ischemia injury model to induce PSC activation and expansion, we show a substantial increase in Raldh1+/Raldh2+ PSCs and Raldh1+ activated macrophages in the lesion core. We find that RA levels are significantly elevated in the ischemic hemisphere and induce signaling in astrocytes and neurons in the peri-infarct region.
Conclusions: This study highlights a dual role for activated, non-neural cells where PSCs deposit fibrotic ECM proteins and, along with macrophages, act as a potentially important source of RA, a potent signaling molecule that could influence recovery events in a neuroprotective fashion following brain injury.
Keywords: Brain fibrosis; Meninges; Pericyte; Perivascular stromal cell; Retinoic acid; Stroke.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

