Keratin impact on PKCδ- and ASMase-mediated regulation of hepatocyte lipid raft size - implication for FasR-associated apoptosis
- PMID: 27422101
- PMCID: PMC5047704
- DOI: 10.1242/jcs.171124
Keratin impact on PKCδ- and ASMase-mediated regulation of hepatocyte lipid raft size - implication for FasR-associated apoptosis
Abstract
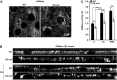
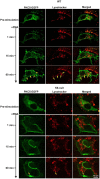
Keratins are epithelial cell intermediate filament (IF) proteins that are expressed as pairs in a cell-differentiation-regulated manner. Hepatocytes express the keratin 8 and 18 pair (denoted K8/K18) of IFs, and a loss of K8 or K18, as in K8-null mice, leads to degradation of the keratin partner. We have previously reported that a K8/K18 loss in hepatocytes leads to altered cell surface lipid raft distribution and more efficient Fas receptor (FasR, also known as TNFRSF6)-mediated apoptosis. We demonstrate here that the absence of K8 or transgenic expression of the K8 G62C mutant in mouse hepatocytes reduces lipid raft size. Mechanistically, we find that the lipid raft size is dependent on acid sphingomyelinase (ASMase, also known as SMPD1) enzyme activity, which is reduced in absence of K8/K18. Notably, the reduction of ASMase activity appears to be caused by a less efficient redistribution of surface membrane PKCδ toward lysosomes. Moreover, we delineate the lipid raft volume range that is required for an optimal FasR-mediated apoptosis. Hence, K8/K18-dependent PKCδ- and ASMase-mediated modulation of lipid raft size can explain the more prominent FasR-mediated signaling resulting from K8/K18 loss. The fine-tuning of ASMase-mediated regulation of lipid rafts might provide a therapeutic target for death-receptor-related liver diseases.
Keywords: ASMase; FasR; Hepatocyte; Keratin; Lipid raft; PKC.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





Similar articles
-
Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes.Apoptosis. 2012 Aug;17(8):880-94. doi: 10.1007/s10495-012-0733-2. Apoptosis. 2012. PMID: 22585043
-
Keratins modulate hepatic cell adhesion, size and G1/S transition.Exp Cell Res. 2007 Jan 1;313(1):179-94. doi: 10.1016/j.yexcr.2006.10.007. Epub 2006 Oct 13. Exp Cell Res. 2007. PMID: 17112511
-
Keratin 8/18 regulation of insulin receptor signaling and trafficking in hepatocytes through a concerted phosphoinositide-dependent Akt and Rab5 modulation.FASEB J. 2017 Aug;31(8):3555-3573. doi: 10.1096/fj.201700036R. Epub 2017 Apr 25. FASEB J. 2017. PMID: 28442548
-
Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies.Hepatology. 2007 Nov;46(5):1639-49. doi: 10.1002/hep.21976. Hepatology. 2007. PMID: 17969036 Review.
-
Revealing the Roles of Keratin 8/18-Associated Signaling Proteins Involved in the Development of Hepatocellular Carcinoma.Int J Mol Sci. 2021 Jun 15;22(12):6401. doi: 10.3390/ijms22126401. Int J Mol Sci. 2021. PMID: 34203895 Free PMC article. Review.
Cited by
-
Sphingomyelinases and Liver Diseases.Biomolecules. 2020 Oct 30;10(11):1497. doi: 10.3390/biom10111497. Biomolecules. 2020. PMID: 33143193 Free PMC article. Review.
-
Regulation of Death Receptor Signaling by S-Palmitoylation and Detergent-Resistant Membrane Micro Domains-Greasing the Gears of Extrinsic Cell Death Induction, Survival, and Inflammation.Cancers (Basel). 2021 May 21;13(11):2513. doi: 10.3390/cancers13112513. Cancers (Basel). 2021. PMID: 34063813 Free PMC article. Review.
-
The role of lipid raft translocation of prohibitin in regulation of Akt and Raf-protected apoptosis of HaCaT cells upon ultraviolet B irradiation.Mol Carcinog. 2017 Jul;56(7):1789-1797. doi: 10.1002/mc.22636. Epub 2017 Mar 6. Mol Carcinog. 2017. PMID: 28218425 Free PMC article.
-
Synergism of rMV-Hu191 with cisplatin to treat gastric cancer by acid sphingomyelinase-mediated apoptosis requiring integrity of lipid raft microdomains.Gastric Cancer. 2021 Nov;24(6):1293-1306. doi: 10.1007/s10120-021-01210-8. Epub 2021 Jul 12. Gastric Cancer. 2021. PMID: 34251544 Free PMC article.
-
Epithelial Keratins Modulate cMet Expression and Signaling and Promote InlB-Mediated Listeria monocytogenes Infection of HeLa Cells.Front Cell Infect Microbiol. 2018 May 14;8:146. doi: 10.3389/fcimb.2018.00146. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868502 Free PMC article.
References
-
- Baribault H., Penner J., Iozzo R. V. and Wilson-Heiner M. (1994). Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8, 2964-2973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

