Endogenous rhythm and pattern-generating circuit interactions in cockroach motor centres
- PMID: 27422902
- PMCID: PMC5051644
- DOI: 10.1242/bio.018705
Endogenous rhythm and pattern-generating circuit interactions in cockroach motor centres
Abstract
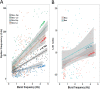
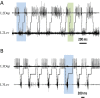
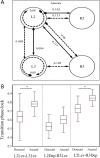
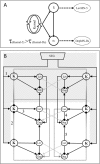
Cockroaches are rapid and stable runners whose gaits emerge from the intricate, and not fully resolved, interplay between endogenous oscillatory pattern-generating networks and sensory feedback that shapes their rhythmic output. Here we studied the endogenous motor output of a brainless, deafferented preparation. We monitored the pilocarpine-induced rhythmic activity of levator and depressor motor neurons in the mesothoracic and metathoracic segments in order to reveal the oscillatory networks' architecture and interactions. Data analyses included phase relations, latencies between and overlaps of rhythmic bursts, spike frequencies, and the dependence of these parameters on cycle frequency. We found that, overall, ipsilateral connections are stronger than contralateral ones. Our findings revealed asymmetries in connectivity among the different ganglia, in which meta-to-mesothoracic ascending coupling is stronger than meso-to-metathoracic descending coupling. Within-ganglion coupling between the metathoracic hemiganglia is stronger than that in the mesothoracic ganglion. We also report differences in the role and mode of operation of homologue network units (manifested by levator and depressor nerve activity). Many observed characteristics are similar to those exhibited by intact animals, suggesting a dominant role for feedforward control in cockroach locomotion. Based on these data we posit a connectivity scheme among components of the locomotion pattern generating system.
Keywords: Central pattern generator; Cockroach; Connectivity model; Extracellular-recording; Locomotion control.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Ayali A., Borgmann A., Büschges A., Couzin-Fuchs E., Daun-Gruhn S. and Holmes P. (2015b). The comparative investigation of the stick insect and cockroach models in the study of insect locomotion. Curr. Opin. Insect Sci. 12, 1-10. 10.1016/j.cois.2015.07.004 - DOI
-
- Bässler U. and Wegner U. (1983). Motor output of the denervated thoracic ventral nerve cord in the stick insect Carausius morosus. J. Exp. Biol. 105, 127-145.
-
- Becht G., Hoyle G. and Usherwood P. N. R. (1960). Neuromuscular transmission in the coxal muscles of the cockroach. J. Insect Physiol. 4, 191-201. 10.1016/0022-1910(60)90026-3 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

