Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart
- PMID: 27422983
- PMCID: PMC5142185
- DOI: 10.1152/ajpheart.00142.2016
Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart
Abstract
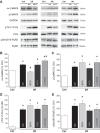
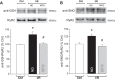
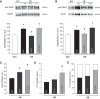
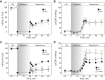
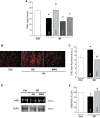
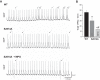
Previous results from our laboratory showed that phosphorylation of ryanodine receptor 2 (RyR2) by Ca(2+) calmodulin-dependent kinase II (CaMKII) was a critical but not the unique event responsible for the production of reperfusion-induced arrhythmogenesis, suggesting the existence of other mechanisms cooperating in an additive way to produce these rhythm alterations. Oxidative stress is a prominent feature of ischemia/reperfusion injury. Both CaMKII and RyR2 are proteins susceptible to alteration by redox modifications. This study was designed to elucidate whether CaMKII and RyR2 redox changes occur during reperfusion and whether these changes are involved in the genesis of arrhythmias. Langendorff-perfused hearts from rats or transgenic mice with genetic ablation of CaMKII phosphorylation site on RyR2 (S2814A) were subjected to ischemia-reperfusion in the presence or absence of a free radical scavenger (mercaptopropionylglycine, MPG) or inhibitors of NADPH oxidase and nitric oxide synthase. Left ventricular contractile parameters and monophasic action potentials were recorded. Oxidation and phosphorylation of CaMKII and RyR2 were assessed. Increased oxidation of CaMKII during reperfusion had no consequences on the level of RyR2 phosphorylation. Avoiding the reperfusion-induced thiol oxidation of RyR2 with MPG produced a reduction in the number of arrhythmias and did not modify the contractile recovery. Conversely, selective prevention of S-nitrosylation and S-glutathionylation of RyR2 was associated with higher numbers of arrhythmias and impaired contractility. In S2814A mice, treatment with MPG further reduced the incidence of arrhythmias. Taken together, the results suggest that redox modification of RyR2 synergistically with CaMKII phosphorylation modulates reperfusion arrhythmias.
Keywords: arrhythmias; ischemia/reperfusion; redox modifications; ryanodine receptor type 2.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Bell JR, Raaijmakers AJ, Curl CL, Reichelt ME, Harding TW, Bei A, Ng DC, Erickson JR, Vila Petroff M, Harrap SB, Delbridge LM. Cardiac CaMKIIδ splice variants exhibit target signaling specificity and confer sex-selective arrhythmogenic actions in the ischemic-reperfused heart. Int J Cardiol 181: 288–296, 2015. - PubMed
-
- Carmeliet E. Cardiac ionic currents and acute ischemia: From channels to arrhythmias. Physiol Rev 79: 917–1017, 1999. - PubMed
-
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119: 1940–1951, 2009. - PMC - PubMed
-
- Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, Bers DM, Mohler PJ, Ziolo MT, Shannon TR. Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS One 9 e87495, 2014. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

