The von Hippel-Lindau Chuvash mutation in mice alters cardiac substrate and high-energy phosphate metabolism
- PMID: 27422990
- PMCID: PMC5142182
- DOI: 10.1152/ajpheart.00912.2015
The von Hippel-Lindau Chuvash mutation in mice alters cardiac substrate and high-energy phosphate metabolism
Abstract
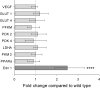
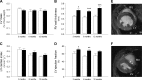
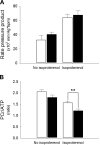
Hypoxia-inducible factor (HIF) appears to function as a global master regulator of cellular and systemic responses to hypoxia. HIF pathway manipulation is of therapeutic interest; however, global systemic upregulation of HIF may have as yet unknown effects on multiple processes. We used a mouse model of Chuvash polycythemia (CP), a rare genetic disorder that modestly increases expression of HIF target genes in normoxia, to understand what these effects might be within the heart. An integrated in and ex vivo approach was employed. Compared with wild-type controls, CP mice had evidence (using in vivo magnetic resonance imaging) of pulmonary hypertension, right ventricular hypertrophy, and increased left ventricular ejection fraction. Glycolytic flux (measured using [(3)H]glucose) in the isolated contracting perfused CP heart was 1.8-fold higher. Net lactate efflux was 1.5-fold higher. Furthermore, in vivo (13)C-magnetic resonance spectroscopy (MRS) of hyperpolarized [(13)C1]pyruvate revealed a twofold increase in real-time flux through lactate dehydrogenase in the CP hearts and a 1.6-fold increase through pyruvate dehydrogenase. (31)P-MRS of perfused CP hearts under increased workload (isoproterenol infusion) demonstrated increased depletion of phosphocreatine relative to ATP. Intriguingly, no changes in cardiac gene expression were detected. In summary, a modest systemic dysregulation of the HIF pathway resulted in clear alterations in cardiac metabolism and energetics. However, in contrast to studies generating high HIF levels within the heart, the CP mice showed neither the predicted changes in gene expression nor any degree of LV impairment. We conclude that the effects of manipulating HIF on the heart are dose dependent.
Keywords: hyperpolarized pyruvate; hypoxia-inducible factor; magnetic resonance imaging.
Copyright © 2016 the American Physiological Society.
Figures


Similar articles
-
The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice.J Clin Invest. 2010 Mar;120(3):827-39. doi: 10.1172/JCI36362. Epub 2010 Feb 8. J Clin Invest. 2010. PMID: 20197624 Free PMC article.
-
Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism.J Mol Med (Berl). 2013 Jan;91(1):59-67. doi: 10.1007/s00109-012-0961-5. Epub 2012 Sep 27. J Mol Med (Berl). 2013. PMID: 23015148 Free PMC article.
-
Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases.Blood. 2021 May 6;137(18):2509-2519. doi: 10.1182/blood.2020009138. Blood. 2021. PMID: 33512384 Free PMC article.
-
Heritable disorders of oxygen sensing.Am J Med Genet A. 2021 Nov;185(11):3334-3339. doi: 10.1002/ajmg.a.62521. Am J Med Genet A. 2021. PMID: 34655169 Review.
-
HIF hydroxylase pathways in cardiovascular physiology and medicine.Circ Res. 2015 Jun 19;117(1):65-79. doi: 10.1161/CIRCRESAHA.117.305109. Circ Res. 2015. PMID: 26089364 Free PMC article. Review.
Cited by
-
Cardiac 31P MR spectroscopy: development of the past five decades and future vision-will it be of diagnostic use in clinics?Heart Fail Rev. 2023 Mar;28(2):485-532. doi: 10.1007/s10741-022-10287-x. Epub 2022 Nov 24. Heart Fail Rev. 2023. PMID: 36427161 Review.
-
Translational repression of HIF2α expression in mice with Chuvash polycythemia reverses polycythemia.J Clin Invest. 2018 Apr 2;128(4):1317-1325. doi: 10.1172/JCI97684. Epub 2018 Feb 26. J Clin Invest. 2018. PMID: 29480820 Free PMC article.
-
Therapeutic targeting of the HIF oxygen-sensing pathway: Lessons learned from clinical studies.Exp Cell Res. 2017 Jul 15;356(2):160-165. doi: 10.1016/j.yexcr.2017.05.004. Epub 2017 May 5. Exp Cell Res. 2017. PMID: 28483447 Free PMC article. Review.
-
Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13122-13130. doi: 10.1073/pnas.1822010116. Epub 2019 May 31. Proc Natl Acad Sci U S A. 2019. PMID: 31152133 Free PMC article.
-
Cardio-onco-metabolism: metabolic remodelling in cardiovascular disease and cancer.Nat Rev Cardiol. 2022 Jun;19(6):414-425. doi: 10.1038/s41569-022-00698-6. Epub 2022 Apr 19. Nat Rev Cardiol. 2022. PMID: 35440740 Free PMC article. Review.
References
-
- Adrogue JV, Sharma S, Ngumbela K, Essop MF, Taegtmeyer H. Acclimatization to chronic hypobaric hypoxia is associated with a differential transcriptional profile between the right and left ventricle. Mol Cell Biochem 278: 71–78, 2005. - PubMed
-
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32: 614–621, 2002. - PubMed
-
- Bakermans AJ, Dodd MS, Nicolay K, Prompers JJ, Tyler DJ, Houten SM. Myocardial energy shortage and unmet anaplerotic needs in the fasted long-chain acyl-CoA dehydrogenase knockout mouse. Cardiovasc Res 100: 441–449, 2013. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

