The Clinical Impact and Cost Effectiveness of Quadrivalent Versus Trivalent Influenza Vaccination in Finland
- PMID: 27423657
- PMCID: PMC4980401
- DOI: 10.1007/s40273-016-0430-z
The Clinical Impact and Cost Effectiveness of Quadrivalent Versus Trivalent Influenza Vaccination in Finland
Abstract
Background: Trivalent influenza vaccines encompass one influenza B lineage; however, predictions have been unreliable on which of two antigenically distinct circulating lineages will dominate. Quadrivalent seasonal influenza vaccines contain strains from both lineages. This analysis assesses the cost effectiveness of switching from trivalent inactivated influenza vaccination (TIV) in Finland to quadrivalent vaccination, using inactivated (QIV) or live-attenuated (Q-LAIV) vaccines.
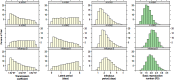
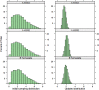
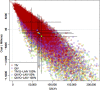
Methods: A transmission model simulated the dynamics of influenza infection while accounting for indirect (herd) protection. Prior distributions for key transmission parameters were repeatedly sampled and simulations that fitted the available information on influenza in Finland were recorded. The resulting posterior parameter distributions were used in a probabilistic sensitivity analysis in which economic parameters were sampled, simultaneously encompassing uncertainty in the transmission and economic parameters. The cost effectiveness of a range of trivalent and quadrivalent vaccine policies over a 20-year time horizon was assessed from both a societal and payer perspective in 2014 Euros.
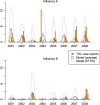
Results: The simulated temporal incidence pattern of symptomatic infections corresponded well with case surveillance data. A switch from the current TIV to Q-LAIV in children (2 to <18 years) and to QIV in other ages was estimated to annually avert approximately 76,100 symptomatic infections (95 % range 36,700-146,700), 11,500 primary care consultations (6100-20,000), 540 hospitalisations (240-1180), and 72 deaths (32-160), and was cost-saving relative to TIV (€374 million averted [€161-€752], in 2014 Euros, discounted at 3 %). This scenario had the highest probability of being the most cost-effective scenario considered.
Conclusions: This analysis demonstrates that quadrivalent vaccination is expected to be highly cost effective, reducing the burden of influenza-related disease.
Figures


Similar articles
-
Cost-effectiveness evaluation of quadrivalent influenza vaccines for seasonal influenza prevention: a dynamic modeling study of Canada and the United Kingdom.BMC Infect Dis. 2015 Oct 27;15:465. doi: 10.1186/s12879-015-1193-4. BMC Infect Dis. 2015. PMID: 26503131 Free PMC article.
-
Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy.Hum Vaccin Immunother. 2018;14(8):1867-1873. doi: 10.1080/21645515.2018.1469368. Epub 2018 Jun 18. Hum Vaccin Immunother. 2018. PMID: 29708843 Free PMC article.
-
Impact of quadrivalent influenza vaccines in Brazil: a cost-effectiveness analysis using an influenza transmission model.BMC Public Health. 2020 Sep 9;20(1):1374. doi: 10.1186/s12889-020-09409-7. BMC Public Health. 2020. PMID: 32907562 Free PMC article.
-
Review of seasonal influenza in Canada: Burden of disease and the cost-effectiveness of quadrivalent inactivated influenza vaccines.Hum Vaccin Immunother. 2017 Apr 3;13(4):867-876. doi: 10.1080/21645515.2016.1251537. Epub 2016 Nov 18. Hum Vaccin Immunother. 2017. PMID: 27858509 Free PMC article. Review.
-
Inactivated influenza virus vaccines: the future of TIV and QIV.Curr Opin Virol. 2017 Apr;23:102-106. doi: 10.1016/j.coviro.2017.04.005. Epub 2017 May 12. Curr Opin Virol. 2017. PMID: 28505524 Free PMC article. Review.
Cited by
-
Betting on the fastest horse: Using computer simulation to design a combination HIV intervention for future projects in Maharashtra, India.PLoS One. 2017 Sep 5;12(9):e0184179. doi: 10.1371/journal.pone.0184179. eCollection 2017. PLoS One. 2017. PMID: 28873452 Free PMC article.
-
A review of the value of quadrivalent influenza vaccines and their potential contribution to influenza control.Hum Vaccin Immunother. 2017 Jul 3;13(7):1640-1652. doi: 10.1080/21645515.2017.1313375. Epub 2017 May 22. Hum Vaccin Immunother. 2017. PMID: 28532276 Free PMC article. Review.
-
Influenza Vaccination for the Prevention of Cardiovascular Disease in the Americas: Consensus document of the Inter-American Society of Cardiology and the Word Heart Federation.Glob Heart. 2021 Aug 5;16(1):55. doi: 10.5334/gh.1069. eCollection 2021. Glob Heart. 2021. PMID: 34381676 Free PMC article. Review.
-
Cost-Effectiveness Analysis of Switching from Trivalent to Quadrivalent Seasonal Influenza Vaccine in Argentina.Vaccines (Basel). 2021 Apr 1;9(4):335. doi: 10.3390/vaccines9040335. Vaccines (Basel). 2021. PMID: 33916048 Free PMC article.
-
Optimising age coverage of seasonal influenza vaccination in England: A mathematical and health economic evaluation.PLoS Comput Biol. 2020 Oct 6;16(10):e1008278. doi: 10.1371/journal.pcbi.1008278. eCollection 2020 Oct. PLoS Comput Biol. 2020. PMID: 33021983 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

